Imaging of Bone ECM hydrogels in their hydrated state for regenerative medicine applications
- Abstract number
- 423
- Presentation Form
- Contributed Talk
- DOI
- 10.22443/rms.mmc2023.423
- Corresponding Email
- [email protected]
- Session
- Cryo-Electron Microscopy
- Authors
- Mr Andreas Rialas (1), Mr Joshua Jones (1), Dr Lisa White (1), Dr Christopher Parmenter (1)
- Affiliations
-
1. University of Nottingham
- Keywords
Imaging, Hydrogels, Hydrated state, Critical Point Drying, Freeze Drying, Slush Freezing
- Abstract text
Summary
Current hydrogel characterisation techniques can result in morphological alterations of the structure and as such other techniques have to be explored as alternatives. Here we demonstrate that techniques such as freeze drying, slush freezing and critical point drying induce various structural alterations and artefacts, suggesting that a different approach should be to characterise hydrogels as close to their hydrated state.
Introduction
Hydrogels are becoming increasingly important in the biomedical and materials science industries. As such, it is essential to understand how they can be characterized and analysed accurately. Current popular imaging modalities used such as scanning electron microscopy (SEM) and transmission electron microscopy (TEM) often require the removal of water which can result in the characterisation of hydrogels with non-native structures. To minimise these potential drying effects, cryogenic sample preparation and characterisation techniques have been investigated, including a variety of techniques, such as slush freezing, to preserve the structures and evaluate them. The purpose of these methods is to preserve the native structures and features of the gels, which may be destroyed by traditional methods such as freeze-drying or chemical fixation. This work should allow for an in-depth understanding of hydrogel behaviour and properties, which can be used for improved product design and development in the future.
Methodology
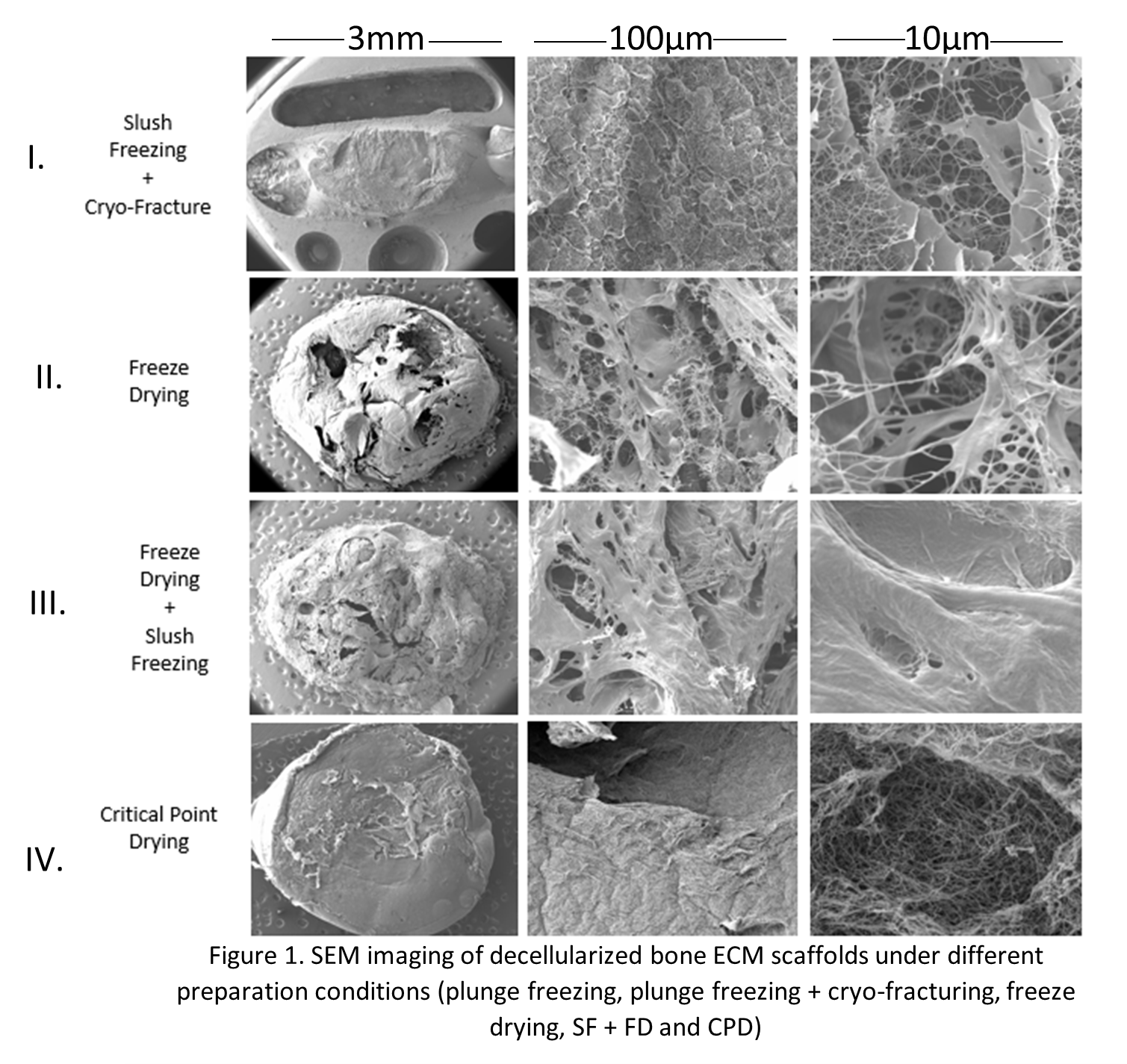
Decellularized bone Extracellular Matrix Gels (ECM) hydrogels were generated (Sawkins et al. 2013) and prepared via different conditions (Freeze-drying, critical point drying, slush freezing and slush freezing followed by freeze drying (SF+FD)) and imaged under room temperature SEM and cryo-SEM.
Results and Discussion
SEM and cryo-SEM performed on bone derived ECM hydrogels revealed that sample preparation performed through slush freezing (I), freeze drying (II) and SF+FD (III) resulted in samples saturated with artefacts such as ice-crystals, distorted surfaces with cracking, folding or holes and altered fibre morphology such as shrinking. In addition, the slush frozen samples required cryo-fracturing to view the internal fibre structure whilst in the freeze-dried samples, the structural morphology was viewed through cracks on the surface of the structure. Samples prepared through CPD (IV) displayed no ice crystal artefacts or major overall sample distortion. In addition the fibre network could be viewed without the need of cryo-fracture or as in the FD samples by looking into cracks in the surface. CPD preparation is performed using ethanol replacement, meaning that the gels may not be considered to be a hydrated ‘native’ state due to the replacement of their main component, water.
Conclusion
In conclusion, the use of preparation techniques such as FD, SF, SF + FD and CPD for the characterisation of bone ECM hydrogels results in morphological alterations of hydrogels as well as the induction of artefacts. Furthermore, even if CPD reduces the formation of some of these artefacts, it involves the removal of water which is a major component of these systems and as such characterisation of gels using this technique can’t be considered hydrated ‘native’ state.
- References
Sawkins, M.J. et al. (2013) “Hydrogels derived from demineralized and decellularized bone extracellular matrix,” Acta Biomaterialia, 9(8), pp. 7865–7873. Available at: https://doi.org/10.1016/j.actbio.2013.04.029.