Understanding Zn Dendrite Growth in Different Aqueous Electrolytes by in situ liquid cell TEM
- Abstract number
- 279
- Presentation Form
- Poster
- Corresponding Email
- [email protected]
- Session
- Poster Session One
- Authors
- Ms Yi Yuan (1), Mr/Dr Shengda Pu (1), Ms Zixuan Li (1), Mr/Dr Xiangwen Gao (1), Mr/Dr Alex Robertson (2)
- Affiliations
-
1. University of Oxford
2. University of Warwick
- Keywords
In situ liquid cell, Zn metal batteries, Zn dendrites, nucleation and growth, electrolyte additives
- Abstract text
Rechargeable metal batteries have been considered as one of the most promising techniques to meet the fast-growing demand of high-energy-density battery systems for modern electrical devices. Zn metal batteries (ZMBs) are among strong candidates due to their relatively high theoretical capacity (820 mAh g-1), lower cost and better safety. Especially, the stability of Zn metal in water endows ZMBs with capability of usage in aqueous electrolytes, which makes them nontoxic and more eco-friendly. Practical application of aqueous ZMBs is seriously suffering from the severe growth of Zn dendrites. The deposition and stripping process on the Zn metal anode is mainly dominated by the mass transfer, the surface polarization and the 2D diffusion of ions on the electrode, which leads to an intrinsic tendency to form protrusions. This will further result in an uneven growth of Zn and the formation of dendrites due to the ‘tip effect’, which implies the preferential accumulation of ions on the higher tips and the vertical growth of protrusions.
Many strategies have been developed to solve this problem and adding additives into electrolytes is found to be one of most facile and effective methods. However, the mechanisms of dendrite growth and roles of these additives in directing uniform Zn deposition are not fully understood. Some operando imaging techniques, such as in-situ optical microscopy and X-ray computed tomography, have been utilized to study the growth of Zn dendrites in electrolytes, but the nucleation and initial growth are very difficult to be seen due to the limitation of resolution. Herein, we investigated the electrodeposition behaviours of Zn in different electrolytes at the nanoscale by utilizing the in-situ electrochemical liquid cell transmission electron microscopy (TEM) technique, which makes it possible to study the behaviour of the initial nucleation and growth of Zn particles as well as influences of different electrolyte environments on it.
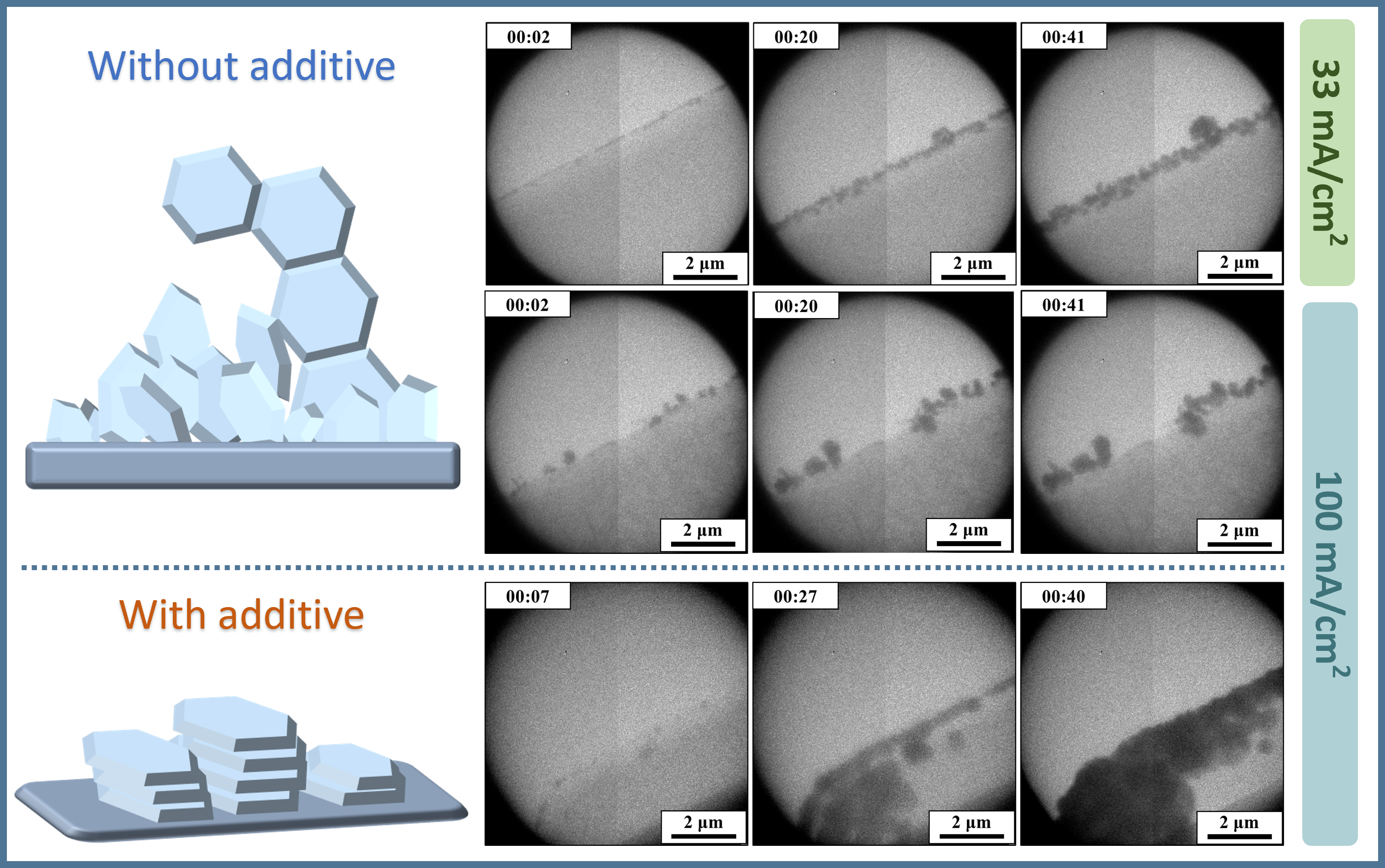
The nucleation and growth of Zn dendrites in 2 M ZnSO4 aqueous electrolyte under different current densities were studied first. It was observed that the Zn particles were generated at the edge of the Pt electrode after the plating started, and gradually grew into Zn flakes which randomly stood on the edge of the electrode. In addition, some of the newly deposited Zn were found to preferably grow from the edges of the previously deposited flakes, evolving into a shape of “daisy-chain” subsequently. This electrodeposition behaviour, resulting from the “tip effect”, reveals a formation mechanism of Zn dendrites in the mild aqueous electrolyte.
LiCl has been reported to be an effective additive to alleviate the formation of zinc dendrites, but the underlying mechanism remains to be unclear. Herein, 2 M LiCl was introduced into the electrolyte as the additive. Interestingly, significant differences in the electrodeposition behaviour were observed, that the deposited Zn flakes aggregated and grew into blocks instead of forming dendrites with the “daisy-chain” structure. It was found that the secondary nucleation of Zn was induced on the (002) facet of preciously deposited Zn hexagonal flakes, instead of the (100) facet which was known to preferentially induce the growth of Zn. This behaviour of the favoured nucleation on the planar surface of the deposited Zn flakes can be explained by the electrostatic shielding effect, that the edges of Zn dendrites were isolated by Li ions adsorbed on them. The Zn||Ti coin cells with LiCl as the additive showed better cycling performances. What’s more, after cycling, the coin cells were disassembled and the separators of the cells with LiCl were found to be much cleaner than those from cells without the additive, illustrating fewer protuberances and side-products on the electrode during cycling. The SEM images of the electrodes after cycling confirmed this electrodeposition preference as well.
In summary, the initial nucleation and the further growth of Zn in different electrolytes at the nanoscale were reveal by the utilization of the in situ liquid cell TEM technique. We have demonstrated the formation process of Zn dendrites resulted from the ‘tip effect’, and the preference of secondary nucleation at the edge of Zn flakes in the commercial 2 M ZnSO4 aqueous electrolyte. Then, with LiCl as the additive, different secondary nucleation behaviour was observed, which showed a tendency of nucleating on the planar (002) face, leading to the formation of Zn blocks instead of Zn dendrites of a ‘daisy chain’ shape. The electrochemical performance of the Zn||Ti cell was improved with LiCl added, showing a higher CE and fewer dendrite residuals in the separators.
- References
- Li, H., Guo, S. and Zhou, H. (2023) ‘Recent advances in manipulating strategy of aqueous electrolytes for Zn anode stabilization’, Energy Storage Materials, 56, pp. 227–257. doi: 10.1016/J.ENSM.2023.01.027.
- Hao, Junnan et al. (2019) ‘Toward High-Performance Hybrid Zn-Based Batteries via Deeply Understanding Their Mechanism and Using Electrolyte Additive’, Advanced Functional Materials, 29(34), p. 1903605. doi: 10.1002/ADFM.201903605.
- Yang, Q. et al. (2020) ‘Dendrites in Zn-Based Batteries’, Advanced Materials, 32(48). doi: 10.1002/ADMA.202001854.
- Guo, X. et al. (2021) ‘Alleviation of Dendrite Formation on Zinc Anodes via Electrolyte Additives’, ACS Energy Letters, 6(2), pp. 395–403. doi: 10.1021/acsenergylett.0c02371.