In-situ cryo 4D-STEM study of biomaterials
- Abstract number
- 446
- Presentation Form
- Poster
- DOI
- 10.22443/rms.mmc2023.446
- Corresponding Email
- [email protected]
- Session
- Poster Session One
- Authors
- Mr. Bing Han (1), Dr. Alexander Eggeman (1), Dr. Chinnawich Phamornnak (1), Dr. Jonny Blaker (1), Mr. Davide Verdolino (1), Prof. Sarah Cartmell (1)
- Affiliations
-
1. University of Manchester
- Keywords
In-situ, Cryo, 4D-STEM, Biomaterials
- Abstract text
Biomaterials have been extensively studied for the recent decades because of their unique properties [1]. For example, collagen matrix with ORC (oxidized regenerated cellulose) have been used for wound healing agent as they have high absorption and hemostatic ability[2], especially the addition of silver-modified collagen can promote nerve growth[3].
However, the TEM/STEM study of biomaterials has been challenging for the last decades because they are energy sensitive[4]. But the use of in-situ cryogenic holder to operate 4D-STEM at ~-170℃ can significantly reduce the damage by the heat energy. This has enabled researchers to obtain TEM/STEM images and sufficient EDX signals for elemental mapping from biomaterials stabilized by the -170℃ environment, greatly increasing the samples’ stability.
This research investigated silk fibroin produced by electrospinning[5] and collagen provided by 3M® company for tissue engineering, and DTX (docetaxel) loaded microbubbles provided by the University of Toronto that are for cancer-treated drug delivery. Experiments were carried out using a Talos F200X TEM equipped with a Merlin diffraction camera and a Gatan ‘Elsa’ cryogenic holder.
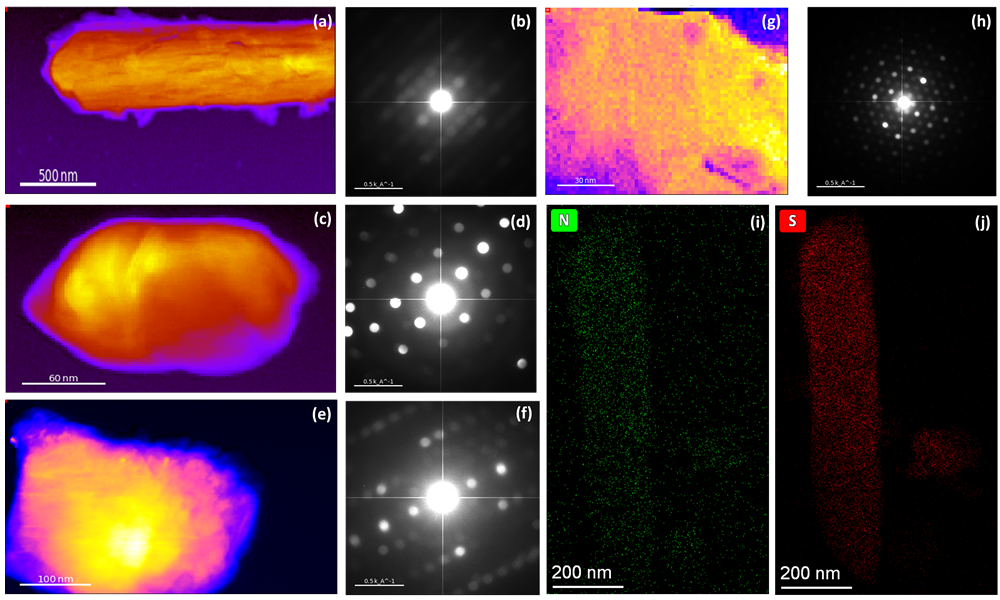
Figure 1. Virtual bright field (VBF) image of DTX-loaded microbubble (a), collagen matrix with ORC (c), collagen matrix with ORC and silver (e), and electrospun silk fibroin (g) with corresponded summed diffraction pattern (b), (d), (f), (h), respectively. Nitrogen (i) and sulphur (j) element mapping of silk fibroin (g) obtained with cryogenic holder under ~-170℃.
The research of DTX-loaded microbubbles (Figure 1a) focused on the crystal structure of DTX drugs recrystallized from the bubbles. Small crystals are more distributed around the sample than in the center for drugs recrystallized out of the bubbles, indicating that the drug may dissolve in the bubbles as several small crystals, which can then be observed in dried shell fragments. By studying the drug that has recrystallized from the lipid systems, we can explore the possibilities of whether the crystal structure or the morphology (such as size and shape) of the material affects its exceptionally high bioactivity.
For collagen, the research focuses on the changes in its microstructure and crystal structure at nanoscale when different components are added. Since type I collagen is closer to amorphous phase[6], the addition of ORC (Figure c) significantly increases the crystallinity of collagen. Besides, for 1% Ag-modified type I collagen (Figure 1e) compared to type I collagen with 45% ORC (Figure 1c), there are diverse changes in morphology and crystal structure, indicating that silver and ORC have a significant influence on both appearance and growth of the samples. The crystal structure study of modified collagen under ~-170℃ would allow a deeper understanding of the growth of the materials and give us an opportunity of crystal phase mapping. In addition, the structural changes of collagen in different dry and wet states are also the focus of future research.
The 4D-STEM diffraction pattern in Figure 1(h) shows that untreated silk (Figure g) has an orthorhombic polytype named silk II after ethanol treatment, which is different from the monoclinic structure of silk I. PEDOT: PSS conductive polymer doping of silk fibroin is confirmed by the overlapping distribution of nitrogen (Figure 1i) and sulfur (Figure 1j) on the EDX mapping. The XPS study indicates that the nitrogen peak in doped silk fibroin shifts after the addition of conductive dopants, with a corresponding change in the sulfur peaks suggesting the formation of new bonds with nitrogen. Silk II can be formed from parallel or anti-parallel amino acid strands, and the incomplete hydrogen bonding in the anti-parallel strand provides unsaturated N-H functionality on the material, which can react with sulfur from PEDOT: PSS. This implies that S-N bonds may be the reaction site for PEDOT: PSS doping, and that the conductive polymers are doped onto the silk fibroin.
These works provide new insights into the biomaterials microstructure and demonstrate the feasibility of using in-situ cryogenic TEM/STEM to study beam sensitive biomaterials and opens new avenues for research in low electron dose area.
- References
- Phamornnak, C., Han, B., Spencer, B. F., Ashton, M. D., Blanford, C. F., Hardy, J. G., ... & Cartmell, S. H. (2022). Instructive electroactive electrospun silk fibroin-based biomaterials for peripheral nerve tissue engineering. Biomaterials Advances, 141, 213094.
- Hart, Jeffrey, et al. "The role of oxidised regenerated cellulose/collagen in wound repair: effects in vitro on fibroblast biology and in vivo in a model of compromised healing." The international journal of biochemistry & cell biology 34.12 (2002): 1557-1570.
- Chowdhry, Saeed A. "Use of oxidized regenerated cellulose (ORC)/collagen/silver-ORC dressings to help manage skin graft donor site wounds." JPRAS open 22 (2019): 33-40.
- S. Inagaki, N. Goto, and K. Yoshikawa. Antibonding delocalization: Geminal Interaction of σ-bonds and Angle Strain. J. Am. Chem. Soc. 1991, 113, 7144-7146.
- Balint, Richard, Nigel J. Cassidy, and Sarah H. Cartmell. "Conductive polymers: Towards a smart biomaterial for tissue engineering." Acta biomaterialia 10.6 (2014): 2341-2353.
- Amirrah, Ibrahim N., et al. "A comprehensive review on collagen type I development of biomaterials for tissue engineering: From biosynthesis to bioscaffold." Biomedicines 10.9 (2022): 2307.