Innovative 3D approach to evaluate extracellular matrix in pancreatic islets using Light Sheet Fluorescence Microscopy
- Abstract number
- 320
- Presentation Form
- Poster & Flash Talk
- DOI
- 10.22443/rms.mmc2023.320
- Corresponding Email
- [email protected]
- Session
- Poster Session Two
- Authors
- Matias Ramirez Acosta (2), Pierre Gianello (2), Caroline Bouzin (1)
- Affiliations
-
1. IREC Imaging Platform / Université catholique de Louvain
2. Laboratory of Experimental Surgery and Transplantation / Université catholique de Louvain
- Keywords
Light sheet fluorescence microscopy
3D image analysis
Pancreatic islets
Type 1 diabetes
Extracellular matrix
Hypoxia
- Abstract text
Summary
We developed a protocol for clearing, multiplex immunofluorescence, 3D light sheet fluorescence microscopy (LSFM) and digital image analysis (LSM) to evaluate changes in pancreatic islets’ extracellular matrix (ECM), in the context of islet transplantation.
Introduction
Pancreatic islets can be transplanted to treat type 1 diabetes mellitus. However, this approach is limited by reduced survival and function of islets, mainly due to hypoxia and anoikis, an apoptotic process triggered by elimination of ECM during islet isolation.
To evaluate the impact of hypoxia on ECM, we developed an innovative imaging approach, based on tissue clearing and 3D microscopy, to visualize and quantify up to four concomitant immunofluorescence (IF) stains. We evaluated three ECM proteins, collagen 4, fibronectin and laminin, and E-cadherin, an adhesion protein involved in anoikis.
Materials and methods
We cultured human islets in normoxic (O2 21%) or hypoxic (O2 1%) conditions. After fixation, they were submitted to an IF protocol consisting in blockage of unspecific epitopes using BSA, followed by 3-day antibody incubations, using different combinations of antibodies targeting insulin, glucagon, carbonic anhydrase 9 (CA9), collagen 4, fibronectin, laminin, E-cadherin, and Hoechst as a nuclear stain. Islets were then cleared using an adaptation of the CUBIC technique, mounted in agarose rods, and imaged using a LSFM. Imaris® was used for 3D rendering, morphological evaluation, and quantification, using ad-hoc protocols.
Results/Discussion
Insulin and glucagon allowed islet identification, and the hypoxia marker CA9 certified hypoxic conditions.
Volumetric analysis of target proteins showed no significant differences amongst experimental groups. However, 3D projections permitted the identification of specific morphological features differentiating islets cultured in normoxic and hypoxic conditions.
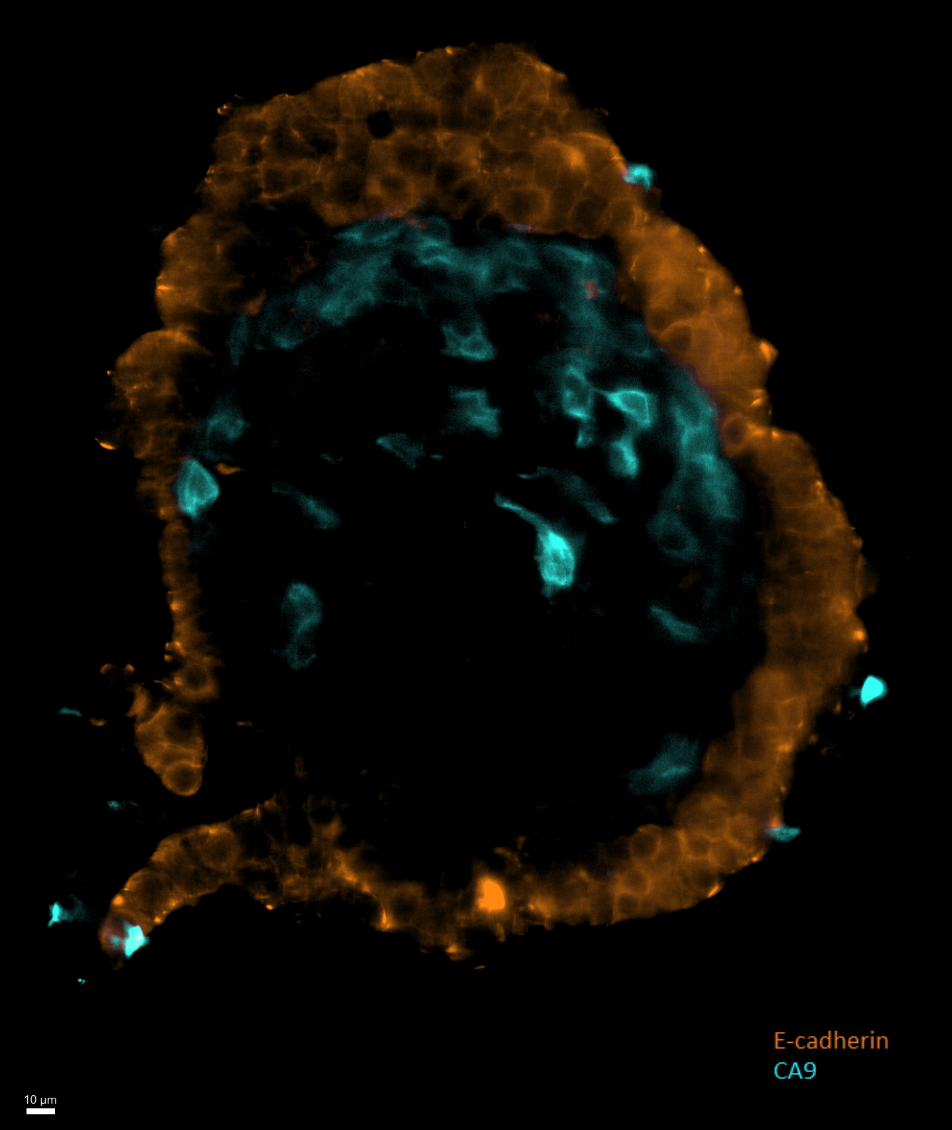
In hypoxia, we could highlight areas or scattered nuclei and clusters of ECM proteins, evoking scarring tissue (Figure 1). Interestingly, E-cadherin was absent in these areas (Figure 2), in agreement with what is described in the literature.
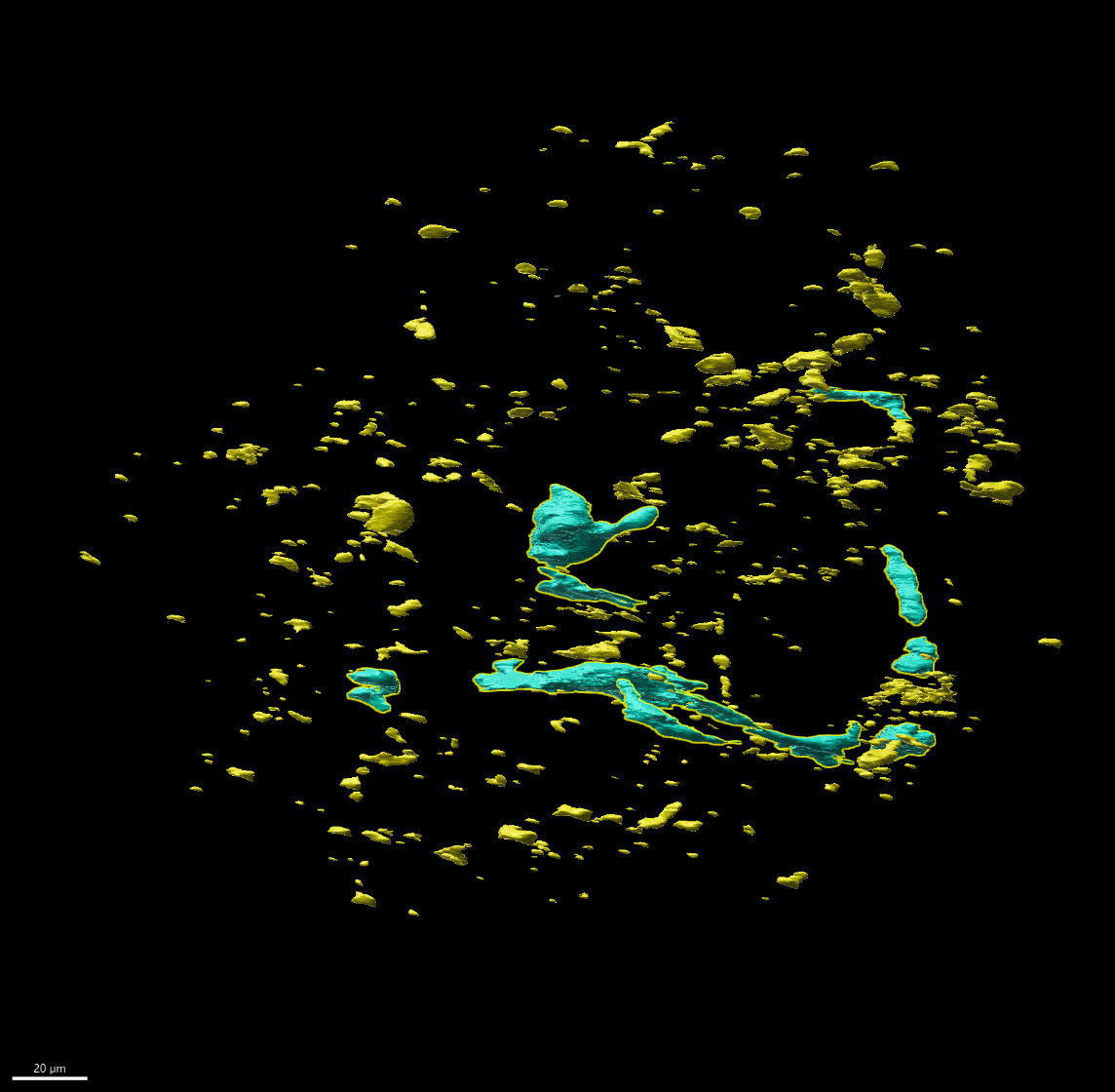
Under normoxic conditions, areas of scarring were also identified, although smaller than in hypoxia. Collagen 4 was found coating blood vessels (Figure 3).
Taken together, our results show that employing a three-dimensional method for morphological evaluation offers comprehensive understanding of protein distribution, revealing distinctions between groups that cannot be grasped through simple total protein quantification. Indeed, hypoxic islets presented similar quantities of proteins than normoxic islets, although their distribution corresponded to tissue scarring. This is in line with the diminution of functional mass shown by transplanted islets, which suffer from a period of low oxygenation immediately after grafting.
Conclusion
Using 3D assessment of islets structure, we highlighted ECM organizational changes during hypoxia, providing morphological evidence of anoikis. This methodology can support the development of new strategies to improve islet transplantation.
Figures
Figure 1. Slice view of an islet, showing a central scar, characterized by condensed fibres of ECM (fibronectin, in green), and scarce nuclei (white), which present altered morphology, like irregular shape and small size.
Figure 2. Slice view of an islet, showing e-cadherin-positive cells (orange), localized in the periphery of the islet. A central accumulation of CA9-positive cells (cyan) suggests a region of cellular hypoxia.
Figure 3 – 3D projection of Collagen 4 IF, where we can identify scattered areas of signal (yellow) and structures consistent with blood vessels (light blue).
- References
Susaki, E. A., Shimizu, C., Kuno, A., Tainaka, K., Li, X., Nishi, K., Morishima, K., Ono, H., Ode, K. L., Saeki, Y., Miyamichi, K., Isa, K., Yokoyama, C., Kitaura, H., Ikemura, M., Ushiku, T., Shimizu, Y., Saito, T., Saido, T. C., Fukayama, M., … Ueda, H. R. (2020). Versatile whole-organ/body staining and imaging based on electrolyte-gel properties of biological tissues. Nature communications, 11(1), 1982. https://doi.org/10.1038/s41467-020-15906-5
Roostalu, U., Lercke Skytte, J., Gravesen Salinas, C., Klein, T., Vrang, N., Jelsing, J., & Hecksher-Sørensen, J. (2020). 3D quantification of changes in pancreatic islets in mouse models of diabetes type I and II. Disease models & mechanisms, 13(12), dmm045351. https://doi.org/10.1242/dmm.045351
Kuwabara, R., Qin, T., Alberto Llacua, L., Hu, S., Boekschoten, M. V., de Haan, B. J., Smink, A. M., & de Vos, P. (2023). Extracellular matrix inclusion in immunoisolating alginate-based microcapsules promotes longevity, reduces fibrosis, and supports function of islet allografts in vivo. Acta biomaterialia, 158, 151–162. https://doi.org/10.1016/j.actbio.2022.12.068
Llacua, L. A., Faas, M. M., & de Vos, P. (2018). Extracellular matrix molecules and their potential contribution to the function of transplanted pancreatic islets. Diabetologia, 61(6), 1261–1272. https://doi.org/10.1007/s00125-017-4524-8
Brandhorst, D., Brandhorst, H., Lee Layland, S., Acreman, S., Schenke-Layland, K., & Johnson, P. R. V. (2022). Basement membrane proteins improve human islet survival in hypoxia: Implications for islet inflammation. Acta biomaterialia, 137, 92–102. https://doi.org/10.1016/j.actbio.2021.10.013
Gan, W. J., Do, O. H., Cottle, L., Ma, W., Kosobrodova, E., Cooper-White, J., Bilek, M., & Thorn, P. (2018). Local Integrin Activation in Pancreatic β Cells Targets Insulin Secretion to the Vasculature. Cell reports, 24(11), 2819–2826.e3. https://doi.org/10.1016/j.celrep.2018.08.035
Zhu, Y., Chen, S., Liu, W., Zhang, L., Xu, F., Hayashi, T., Mizuno, K., Hattori, S., Fujisaki, H., & Ikejima, T. (2021). Collagens I and V differently regulate the proliferation and adhesion of rat islet INS-1 cells through the integrin β1/E-cadherin/β-catenin pathway. Connective tissue research, 62(6), 658–670. https://doi.org/10.1080/03008207.2020.1845321
Irving-Rodgers, H. F., Choong, F. J., Hummitzsch, K., Parish, C. R., Rodgers, R. J., & Simeonovic, C. J. (2014). Pancreatic islet basement membrane loss and remodeling after mouse islet isolation and transplantation: impact for allograft rejection. Cell transplantation, 23(1), 59–72. https://doi.org/10.3727/096368912X659880