Investigation of SEI layer formation on HOPG using SPM
- Abstract number
- 291
- Presentation Form
- Submitted Talk
- DOI
- 10.22443/rms.mmc2021.291
- Corresponding Email
- [email protected]
- Session
- Stream 4 (AFM): SPM Techniques on Energy Materials and Processes
- Authors
- Dr Saisameera Mitta (1, 2), Prof. Dr. Ulrich Stimming (1, 2)
- Affiliations
-
1. Newcastle University
2. The Faraday Institution
- Keywords
in situ atomic force microscopy, Li-ion battery, solid electrolyte interface
- Abstract text
The electrochemical processes that occur at the electrode/electrolyte interfaces play a crucial role for the lithium-ion batteries (LIBs) performance [1,2]. The Solid-Electrolyte Interphase (SEI) formed on the negative electrode (Highly oriented pyrolytic graphite-HOPG) surface, which is described as a product of electrochemical reduction of electrolyte components, shifts the electrode potential into the electrolyte stability window [3] consequently preventing further electrolyte decomposition and stabilizing the electrode/electrolyte interface. The optimal SEI should act as a Li+ -ion conductor and an electronic insulator [4]. In modern lithium-ion batteries, the capacity fade is mainly ascribed to a growth of the SEI. A thorough understanding of the SEI formation mechanisms is crucial as batteries can benefit from a proper SEI, as it can increase their lifetime, cycle life and safety [5].
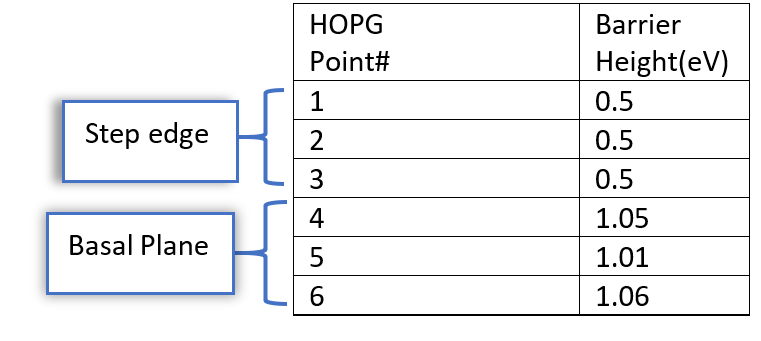
Within this work, we investigated the SEI nucleation and growth on HOPG substrates in 1 M LiPF6 in EC/DEC commercial electrolyte using in situ atomic force microscopy (AFM). We found that the SEI film prefers to nucleate and grow at defective sites like step edges rather than the basal planes. We were able correlate this with the local work functions (effective barrier height). We performed STM current-distance curves which yielded the effective tunnelling barrier. These experiments yielded a difference in barrier height at the step edge and the terrace of 0.5 eV as shown in fig 1.
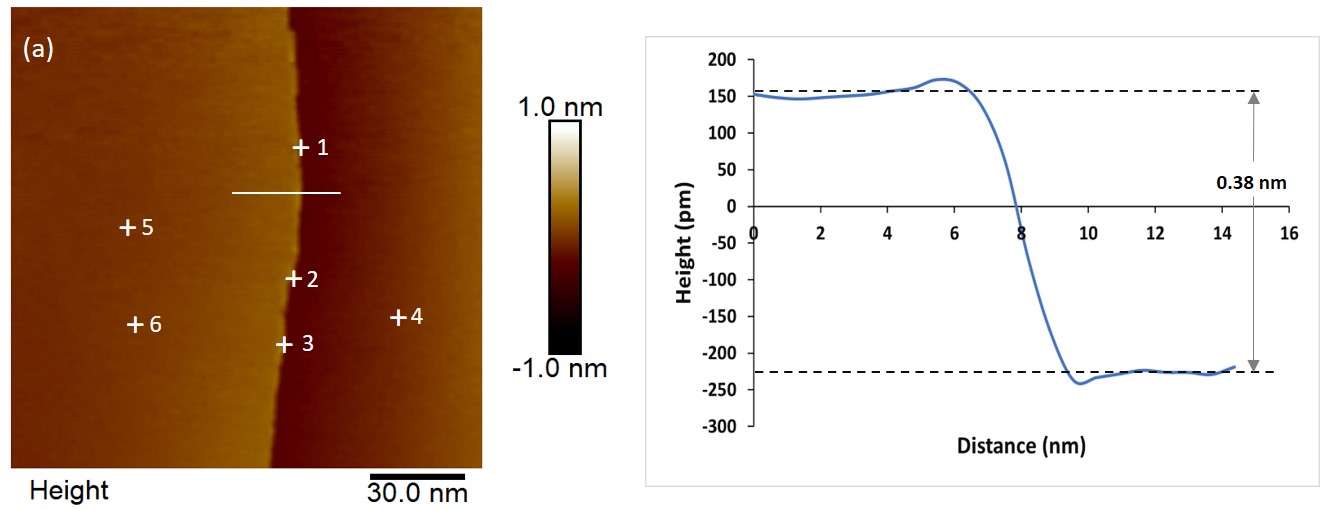
Fig 1.: (a) Constant-current STM image of HOPG (b) Line trace correspond white line in (a) step height is about 0.38nm represents to monoatomic step of HOPG.
Calculated local work functions on step edges and the terraces of freshly cleaved HOPG inside an Argon filled glove box are summarized in the below table.
This difference in barrier height expresses itself in a difference of the local electrochemical potential, with a value at the step edges being lower by 0.5 V as compared to the terrace, the basal plane. This can explain why the SEI layer formation starts at the step edges 0.5 V more negative than on the basal plane.
- References
- Peled, E. The Electrochemical Behavior of Alkali and Alkaline Earth Metals in Nonaqueous Battery Systems-The Solid Electrolyte Interphase Model. J. Electrochem. Soc. 1979, 126, 2047−2051.
- Gauthier, M.; Carney, T. J.; Grimaud, A.; Giordano, L.; Pour, N.; Chang, H. H.; Fenning, D. P.; Lux, S. F.; Paschos, O.; Bauer, C.; Maglia, F.; Lupart, S.; Lamp, P.; Shao-Horn, Y. Electrode-Electrolyte Interface in Li-Ion Batteries: Current Understanding and New Insights. J. Phys. Chem. Lett. 2015, 6 (22), 4653−4672.
- Goodenough, J. B., Manthiram, A. & Wnetrzewski, B. Electrodes for lithium batteries. J. Power Sources 43, 269–275 (1993).
- Seidl, L., Martens, S., Ma, J., Stimming, U. & Schneider, O. In situ scanning tunneling microscopy studies of the SEI formation on graphite electrodes for Li+-ion batteries. Nanoscale 2016, 8, 14004.
- Balbuena, B. and Wang, Y., Lithium-Ion Batteries Solid-Electrolyte Interface, Imperial College Press, 2004.