Investigating the biochemistry of Alzheimer’s disease using synchrotron x-ray microscopy and spectroscopy
- Abstract number
- 176
- Presentation Form
- Poster Flash Talk + Poster
- Corresponding Email
- [email protected]
- Session
- Poster Session 2
- Authors
- James Everett (6, 4), Jake Brooks (4), Frederik Lermyte (4, 7), Vindy Tjendana-Tjhin (4), Germán Plascencia-Villa (1), Ian Hands-Portman (5), Jane Donnelly (4), Kharmen Billimoria (4, 3, 8), George Perry (1), Xiongwei Zhu (2), Peter Sadler (3), Peter O'Connor (3), Joanna Collingwood (4), Neil Telling (6)
- Affiliations
-
1. Department of Biology and Neurosciences Institute, The University of Texas at San Antonio (UTSA)
2. Department of Chemistry, Case Western Reserve University
3. Department of Chemistry, University of Warwick
4. School of Engineering, University of Warwick
5. School of Life Sciences, University of Warwick
6. School of Pharmacy and Bioengineering, Keele University
7. Department of Chemistry, Technical University of Darmstadt
8. LGC Ltd.
- Keywords
Synchrotron, x-rays, microscopy, spectroscopy, metals, neurobiology, neurodegenerative disease, Alzheimer's disease
- Abstract text
Summary: For several decades, disrupted brain metal homeostasis has been linked to the development of dementia-causing neurodegenerative disorders such as Alzheimer’s disease, which afflict millions of individuals worldwide. Despite these observations, our current knowledge of metal biochemistry in neurodegenerative disease lacks the chemical and spatial sensitivity required to understand how metals contribute to disease development. To address these shortcomings, this work employed a variety of hard and soft x-ray synchrotron techniques to characterize the metal biochemistry of Alzheimer’s brain pathologies with exceptional chemical and spatial sensitivity. These experiments demonstrated Alzheimer’s brains to harbour chemically-reactive metal forms previously undocumented in human neurobiology, opening new avenues for disease diagnosis and treatment.
Introduction
Metals are vital for brain well-being. However, when managed inappropriately by the brain, metals can also cause brain damage [1,2]. Brain metal imbalances have been linked to the development of numerous dementia-causing neurodegenerative diseases including Alzheimer’s (AD) and Parkinson’s [3,4], which afflict millions of individuals worldwide. Disruptions to brain metal homeostasis are commonly associated with the formation of pathological protein lesions: an example being the co-localisation of chemically-reduced and potentially toxic iron to Alzheimer’s amyloid-β pathologies [5,6]. Such metal forms are not commonplace in disease-free brains, suggesting their formation to be a result of protein lesion interaction with endogenous metals.
Metal chemistry in biological systems can vary over extremely small (< 100 nm) spatial scales [6]. Thus, to understand these systems, nanoscale resolution imaging and chemical sensitivity is essential. The current knowledge of metal biochemistry in neurodegenerative disease lacks this level of detail required to understand how metals influence disease aetiology; preventing the development of viable metal-targeting technologies for diagnosis and treatment, due to a lack of specificity [7]. To address these shortcomings, this research employed state-of-the-art synchrotron x-ray techniques to establish the nanoscale chemical composition of AD brain tissues and amyloid pathologies.
Materials and Methods
Synchrotron x-ray experiments combined with electron and light microscopies were performed on isolated amyloid plaques/aggregates, cortical tissue from AD subjects, and age-matched disease-free tissue controls.
To map elemental distributions within the samples, x-ray fluorescence (XRF) mapping was performed. For broad distributions across large tissue areas, XRF mapping to a ca. 2 µm spatial resolution was conducted using Diamond Light Source (DLS) microfocus beamline I18. High resolution 100 nm XRF mapping on localized regions of interest (e.g. amyloid plaques) was performed using the hard x-ray nanoprobe on DLS beamline I14. Additional phase contrast ptychography was performed on beamline I14 to visualize nanoscale sample morphology.
To establish the chemical speciation of sample materials at ≤ 50 nm spatial resolution, soft and hard x-ray spectromicroscopy were performed using scanning transmission x-ray microscopy (STXM) at DLS beamline I08 and XANES mapping at beamline I14 respectively. X-ray absorption spectra revealing metal oxidation state and the organic (e.g. C, O and P) composition of the sample material were obtained, allowing the chemical composition of highly localised regions of interest to be determined. Magnetically-sensitive x-ray magnetic circular dichroism (XMCD) STXM measurements were also performed, enabling the magnetic characterisation of both iron compounds and elemental-metallic phases.
No dyes, contrast agents or aldehyde fixatives were used during the preparation of sample materials for synchrotron x-ray analysis, offering an unprecedented insight into the native chemistry of these biological specimens.
Results and Discussion
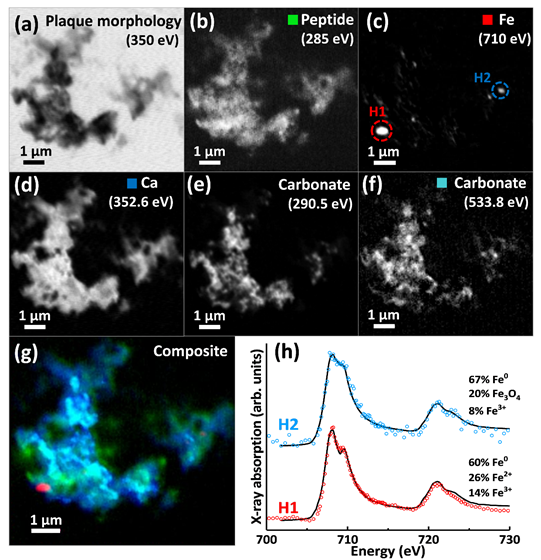
XRF elemental mapping and STXM speciation mapping showed amyloid pathologies from AD tissues to sequester multiple metal elements (e.g. Ca, Fe, Cu, Zn), exceeding metal intensities in the surrounding neuropil where examined. Nanofocus x-ray absorption spectromicroscopy revealed dramatic nanoscale variations in metal oxidation state within the same amyloid plaques, including the discovery of chemically-reduced elemental (zero-oxidation-state) ferromagnetic iron (Fe0) (Figure 1). Metal loading within amyloid plaques corresponded with changes in amyloid organic chemistry, allowing for label-free imaging of amyloid structures against the background tissue. Furthermore, amyloid-β was shown to convert ferric (Fe3+) iron into more reactive chemically-reduced states, including Fe0, directly implicating amyloid-β in the disrupted iron homeostasis observed in AD [8].
The identification of Fe0 within AD amyloid plaques indicates that biogenic metallic elements, previously observed only in microorganisms, viruses and plants [9], can also occur in humans. The reactivity of these metallic phases differs from their metal oxide counterparts, and may redefine our understanding of metal neurochemistry and toxicity in neurodegenerative diseases. Transition metals associated with specific disease pathologies offer potential for disease diagnosis and staging, whilst chemically-reduced iron associated with amyloid structures may represent novel targets for AD therapies intended to lower metal toxicity in affected brain regions.
Conclusions
These results demonstrate the power of combined synchrotron x-ray microscopy and spectroscopy to examine the biochemistry of neurodegenerative disease. The identification of metal types uniquely associated with Alzheimer’s pathology provides a step-change in our understanding of how metals contribute to AD, with potential to facilitate the development of viable therapies designed to restore metal balance in AD brains. The methodology used here can be readily applied to a myriad of biological systems, offering a new toolkit for the examination of biological materials.
Figure 1. STXM examination of a human Alzheimer’s disease amyloid plaque containing metallic iron (Fe0). (a) 350 eV image showing overall plaque morphology. (b) Carbon K-edge protein map. (c) Iron L-edge map. (d) Calcium L-edge map. (e) Carbon K-edge carbonate map. (f) Oxygen K-edge carbonate map (g) Composite image showing: protein (green), calcium (blue), oxygen K-edge carbonate (sky blue), and iron (red) content of the plaque. (h) Iron L2,3-edge x-ray absorption spectra from the iron areas highlighted in (c). Data from Everett et al. Nanoscale (2018) [6].
- References
1. A. I. Bush, Trends Neurosci. 26, 207-214 (2003); 2. J. Prousek, Pure Appl. Chem. 79, 2325-2338 (2007); 3. R. Giampietro, et al., Mol. Pharm. 15, 808-820 (2018); 4. H. Kozlowski, et al., Coord. Chem. Rev. 256, 2129-2141 (2012); 5. C. J. Maynard, et al., Int. J. Exp Pathol. 86, 147-159 (2005); 6. J. Everett, et al., Nanoscale 10, 11782-11796 (2018); 7. M. T. Nuñez and P. Chana-Cuevas, Pharmaceuticals. 11, 109 (2018); 8. J. Everett, et al., Sci Rep. 10, 10332 (2020); 9. J. Huang, et al., Chem. Soc. Rev. 44, 6330-6374 (2015).