Inverted microscope platform for cryo-CLEM and laser-free confocal cryo-fluorescence
- Abstract number
- 230
- Presentation Form
- Poster Flash Talk + Poster
- Corresponding Email
- [email protected]
- Session
- Stream 6 (Frontiers): Correlative Imaging of Organelle Organization and Architecture
- Authors
- Michael Schwertner (2), Phillipa Timmins (1), Kirti Prakash (3), Stephanie Rey (3), Mike Shaw (3)
- Affiliations
-
1. Aurox Ltd.
2. Linkam Scientific Instruments Ltd.
3. National Physical Laboratory
- Keywords
cryo-fluorescence (cryo-FM), cryo-EM, cryo-ET, cryo-CLEM (Correlative Light and Electron Microscopy), cryo-TEM, inverted-upright conversion relay optics
- Abstract text
Cryo-imaging of biological samples embedded in vitrified ice has unique advantages and the development and implementation of cryo-EM and single particle analysis led to a Nobel Prize in 2017 [1]. More recently cryo-EM was also used for research into the structure and mechanisms of the coronavirus [2].
Fluorescence cryo-imaging in confocal and widefield modes can support the electron microscopy workflow in several ways. Firstly, in the form of CLEM (Correlative Light and Electron Microscopy), where fluorescence imaging of samples allows the use of specific and sensitive markers not available in EM. CLEM also limits sample EM beam damage through pre-identification of ROI’s in a light microscope (LM) image with subsequent matching of coordinates. Secondly, cryo-imaging in the LM allows sample pre-screening and assessment of preparation quality saving costly EM time.
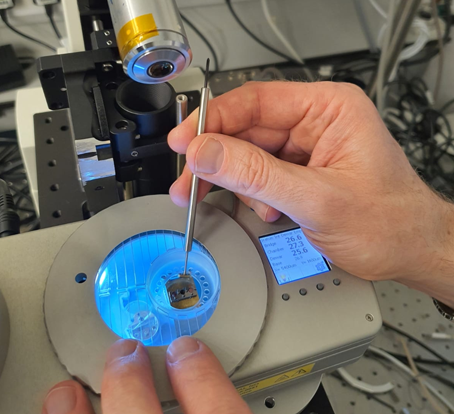
Current cryo-stage designs for fluorescence are typically for upright microscope setups and cannot be integrated with the inverted widefield or confocal fluorescence systems commonly found in bio-labs. While inverted-upright conversion optics are available [3], typical implementations do not allow straightforward sample loading. Here we describe a prototype for the integration of a CMS196V3 (upright) cryo-stage into an inverted microscope system (Nikon Eclipse Ti-E with Aurox Clarity Laser-Free Confocal) with a tilt mechanism for the direct loading of cryo-samples. In this talk we will discuss the hardware integration of the cryo-stage and the Clarity system (see figures 1,2,3), the software integration based on MicroManager (Fiji) [4] and share PSF measurements to assess the optical performance of the conversion system.
Figure 1: Overview of the system on Nikon Ti platform with Linkam CMS196 cryo-stage and Aurox Clarity laser-free confocal unit.
Figure 2: Linkam cryo-stage with engaged tilt-arm.
Figure 3: Optical arm tilted back for sample loading and manipulation.
We anticipate that the convenience of sample loading combined with integration into more common microscope systems and the flexible software architecture can lead to a wider adaptation of cryo-fluorescence and CLEM.
First cryo-confocal images were imaged with the system on HeLa cells marked with GFP and plunge-frozen at NPL on R2/2 gold grids (Quantifoil).
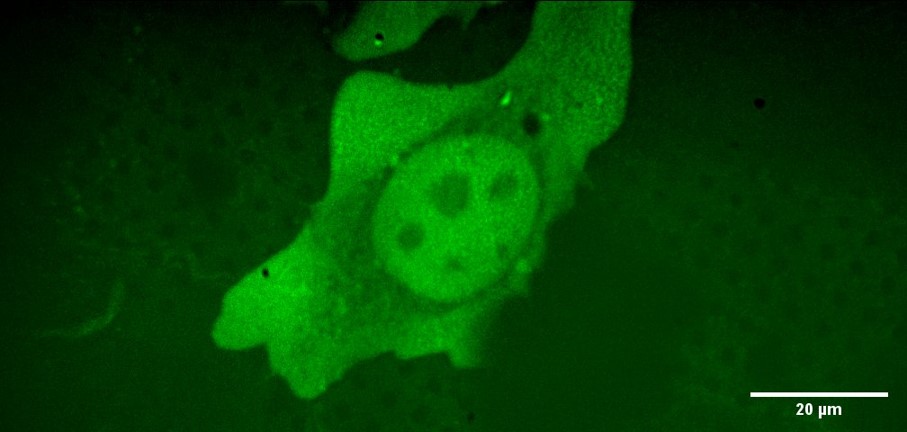
Figure 4 shows a low-magnification cryo-confocal overview (maximum intensity projection) of a sparsely populated grid while figure 5 shows a single cell from the same sample imaged with the 100x NA 0.9 lens under cryo-conditions.
Figure 4: 10x cryo-confocal overview image, HeLa Cells.
Figure 5: Cryo-Confocal image (maximum intensity projection of stack) of HeLa cell with GFP staining using 100x NA 0.9 WD=2.0 mm objective lens and LED illumination (466 nm) coupled to Clarity laser-free confocal unit.
- References
[1] https://www.nature.com/news/cryo-electron-microscopy-wins-chemistry-nobel-1.22738
[2] G. Wolff et al., Science 10.1126/science.abd3629 (2020).
[3] http://lsmtech.com/
[4] https://imagej.net/Fiji