Using the Mesolens to observe structural changes in E. coli mature colony biofilms under different nutrient availability
- Abstract number
- 158
- Presentation Form
- Poster Flash Talk + Poster
- DOI
- 10.22443/rms.mmc2021.158
- Corresponding Email
- [email protected]
- Session
- Stream 5: Imaging in Development and Disease
- Authors
- Ms Beatrice Bottura (1), Prof. Paul Hoskisson (1), Prof. Gail McConnell (1)
- Affiliations
-
1. University of Strathclyde
- Keywords
Biofilms, light microscopy, E. coli
- Abstract text
Escherichia coli exists in planktonic form as rod-shaped cells measuring 1 μm x 2 μm, which can autonomously aggregate in a compact and dynamic structure called a biofilm, held together by a self-secreted extracellular matrix. Biofilms grant protection from external stresses and can lead to antibiotic resistance, and, as such, are a major health concern. A deeper understanding of biofilm structure and growth is needed to tackle these issues.
Bacterial growth is exponential and occurs by binary splitting, but the growth rate and final bacterial biomass depend on growth medium composition - in particular, the type and amount of nutrients available to the cells during growth (Paliy and Gunasekera 2007; Shehata and Marr 1971). Carbon (C) and nitrogen (N) are both essential nutrients for E. coli, and are best provided by glucose and ammonia respectively (Bren et al. 2016). Microbial growth requires an appropriate C/N ratio, and a deviation from this ratio can lead to dramatic changes in the chemical composition of the bacterial surface, in turn affecting bacteria’s ability to adhere to other surfaces (McEldowney & Fletcher, 1985). While biofilm morphology for various levels of nutrients has been investigated by simulations and experiments (Mimura et al. 2000, Matsushita et al. 1990), these studies only investigated the overall 2D topology of the colony, and did not include changes in internal structure at depth.
We have used the Mesolens, a custom-built optical microscope with unusual combination of numerical aperture (0.47) and magnification (4x), to image the structure of whole mature colony biofilms under different nutrient conditions. The Mesolens, which can image a volume of 100 mm3 with subcellular resolution throughout, has previously been used to investigate the internal structure of E. coli biofilms (Rooney et al. 2020), revealing a network of intra-colony channels involved in nutrient uptake. Here we have extended this initial observation to understand the role of carbon and nitrogen nutrient availability in the structure and distribution of intra-colony channels.
We used a non-pathogenic E. coli strain that expressed fluorescence by green fluorescent protein DNA insertion, selected by addition of the antibiotic gentamicin. Cells in mid-exponential growth phase were resuspended in M9 minimal medium with either 10 mM or 80 mM nitrogen (and nominal carbon concentration) or 10 mM or 200 mM carbon (and nominal nitrogen concentration). Cells were inoculated onto M9 agar medium with nutrient concentration matching that of the liquid culture and incubated at 37⁰C for 2 to 3 days (until they reached maturation). Mature colony images were acquired on the Mesolens in both widefield and confocal laser scanning mode, using water immersion for optimum matching of the refractive index of the specimen. Fluorescence was excited by a 490 nm LED (widefield) or a 488 nm laser (confocal), and detected by a sensor-shifting CCD camera or a PMT respectively. FIJI (Schindelin et al. 2019) was used to calculate colony diameter of up to 20 colonies for each nutrient concentration.
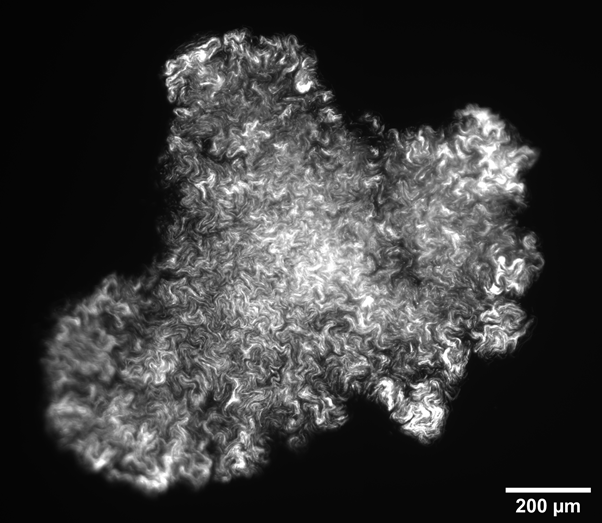
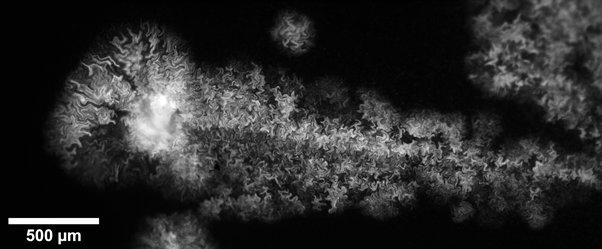
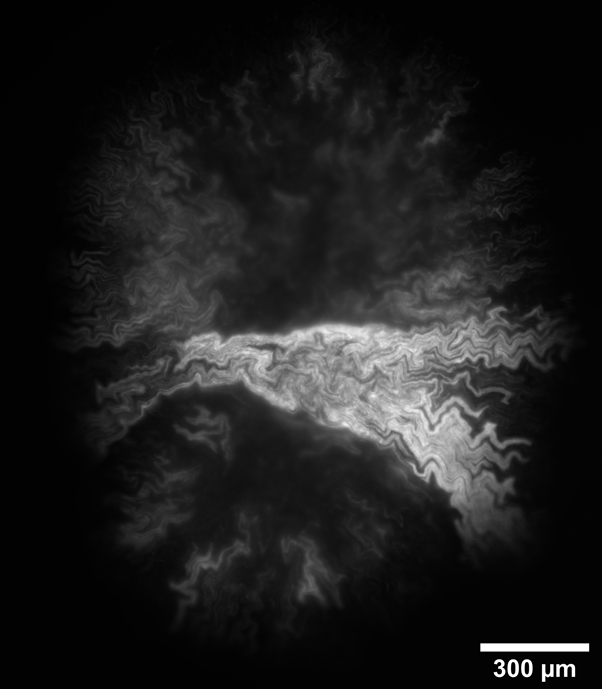
Intra-colony channels were observed under all nutrient conditions, and filled the colony volume entirely. In general, colonies grown on minimal medium substrates were noted to have wider channels than those grown on rich medium. We hypothesise this is because bacterial cells struggle more to extract nutrients from minimal medium than from a rich medium, and hence the channels may have adapted to facilitate nutrient uptake. We found that colonies grown on nutrient-rich substrates were almost double the size than colonies grown on nutrient-deprived conditions. We measured a colony diameter of 2.07 ± 0.15 mm for 200 mM carbon (n=7 colonies) and 1.73 ± 0.17 mm for 80 mM nitrogen (n=7 colonies), while the colonies grown on low-nutrient substrates measured 1.19 ± 0.28 mm for 10 mM nitrogen (n=10 colonies) and 0.69 ± 0.28 mm for 10 mM carbon (n=20 colonies). On low carbon substrates, the colony structure differs greatly, with small, irregularly-shaped aggregates (Figure 1), or into colonies with a linear structure as long as 3 mm (Figure 2). On the other hand, substrates with nitrogen excess led to sectoring (large non-fluorescent portions) (Figure 3). Further work will confirm whether these non-fluorescent regions comprise non-viable cells or are filled with medium.
Figure 1: Colony grown on carbon-deprived substrate displaying irregular shape.
Figure 2: Colony grown on carbon-deprived substrate showing linear colony morphology.
Figure 3: Colony grown on nitrogen excess substrate showing sectoring.
In conclusion, mesoscale imaging allowed us to observe the phenotypical differences brought by varying nutrient concentrations, all while preserving intra-colony channel detail. The high resolution of the acquired images, together with the large volume of capture, enabled the simultaneous study of microscopic and mesoscopic features of bacterial colonies. Measuring the size of whole bacterial colonies revealed a strong dependence of colony size on nutrient availability, and at the same time we confirmed the presence of intra-colony channels at these nutrient concentrations.
- References
- Paliy O, Gunasekera TS. Growth of E. coli BL21 in minimal media with different gluconeogenic carbon sources and salt contents. Appl Microbiol Biotechnol. 2007 Jan;73(5):1169-72
- Shehata TE, Marr AG. Effect of nutrient concentration on the growth of Escherichia coli. J Bacteriol. 1971 Jul;107(1):210-6.
- Bren, A., Park, J., Towbin, B. et al. Glucose becomes one of the worst carbon sources for E.coli on poor nitrogen sources due to suboptimal levels of cAMP. Sci Rep 6, 24834 (2016)
- S. McEldowney, M. Fletcher, Effect of growth conditions and surface characteristics of aquatic bacteria on their attachment to solid surfaces, J. Gen. Microbiol. 132 (1986) 513/523
- Mimura, M. et al. Reaction-diffusion model of bacterial colony patterns,
Phys. A. Stat. Mech. Appl. 2000, 282(1-2):283-3033 - Matsushita, M., Fujikawa, H. Diffusion-limited growth in bacterial colony formation, Phys. A. Stat. Mech. Appl. 1990, 168(1)
- Rooney, L.M., Amos, W.B., Hoskisson, P.A. et al. Intra-colony channels in E. coli function as a nutrient uptake system, ISME J 14, 2461–2473 (2020)
- Schindelin, J., Arganda-Carreras, I., Frise, E. et al. Fiji: an open-source platform for biological-image analysis. Nat Methods 9, 676–682 (2012)