Transverse-tubule remodelling in remote and border regions following myocardial infarction
- Abstract number
- 53
- Presentation Form
- Submitted Talk
- DOI
- 10.22443/rms.mmc2021.53
- Corresponding Email
- [email protected]
- Session
- Stream 3: 3D+ Image Analysis
- Authors
- Miss Tharushi Perera (1), Dr Christian Pinali (1), Dr Emma Radcliffe (1), Miss Barbara Niort (1), Dr Charlene Pius (1), Professor Andrew Trafford (1)
- Affiliations
-
1. University of Manchester
- Keywords
serial block face scanning electron microscopy, transverse-tubule remodelling, myocardial infarction, fragmentation
- Abstract text
Summary
Serial block face scanning electron microscopy (SBF-SEM) was used to image ventricular cardiac myocytes in healthy and myocardial infarction (MI) sheep. 3-dimensional renderings of EM stacks of images revealed an increase of t-tubule remodelling following myocardial infarction, from the remote myocardium to the peri-infarct border zone. This study, thereby increases our understanding of the cardiac ultrastructural remodelling following MI.
Introduction
The highly organised transverse tubule (t-tubule) network consisting of invaginations of the cell sarcolemma facilitates cardiac excitation-contraction coupling and synchronous cardiac myocyte contraction. In heart failure secondary to MI, changes in the structure and organisation of t-tubules result in impaired cardiac contraction. However, there is little knowledge on the regional variation of t-tubule remodelling in cardiac failure. This study aimed to investigate post-myocardial infarction t-tubule remodelling in infarct border and remote regions, in comparison to control, using a translationally relevant ovine ischaemia reperfusion injury (IRI) MI model.
Materials and Methods
Six adult (~18 months) female Welsh Mountain sheep were used in this study (n=3 MI, n=3 control), with all experimental procedures performed in accordance with the United Kingdom (UK) Animals (Scientific procedures) Act 1986 and European Union Directive 2010/63, with local (University of Manchester) Animal Welfare and Ethical Review Board approval. Myocardial infarction was induced using minimally invasive coronary angioplasty of the left anterior descending (LAD) artery (90 minutes), followed by reperfusion. Eight weeks following MI, animals were humanely killed (pentobarbital sodium 200 mg.kg-1; Animalcare Ltd, UK). Small portions (~0.5 to 1 mm3) of left ventricular tissue were collected from the myocardium of control hearts, and from remote and border regions of the infarcted hearts. Tissue samples were fixed in 2.5% glutaraldehyde and 2% paraformaldehyde in 100mM sodium cacodylate buffer, pH 7.2 and stained in 2% osmium tetroxide and 1.5% potassium ferrocyanide in 100nM sodium cacodylate; 1% tannic acid; 1% osmium tetroxide and 1% uranyl acetate with washings in double distilled water following each staining step. Samples were extracted out of the plastic blocks, glued onto cryo pins, sputter-coated in gold-palladium and placed into the Gatan 3View device, hosted inside a Quanta 250 FEG scanning electron microscope (SEM). A stack of images was generated at a nominal voxel size of 8 - 11 nm (x, y) by 50 nm (z). 3dmod was used to visualise the stack of images and segment the t-tubule network for control, remote and border regions. Numerical information was extracted from the 3dmod model files and analysed using Microsoft Excel and GraphPad Prism. A one-way ANOVA test with a Tukey’s multiple comparison test was performed on normally distributed data, and a Mann-Whitney U test was carried out on skewed data.
Results and Discussion
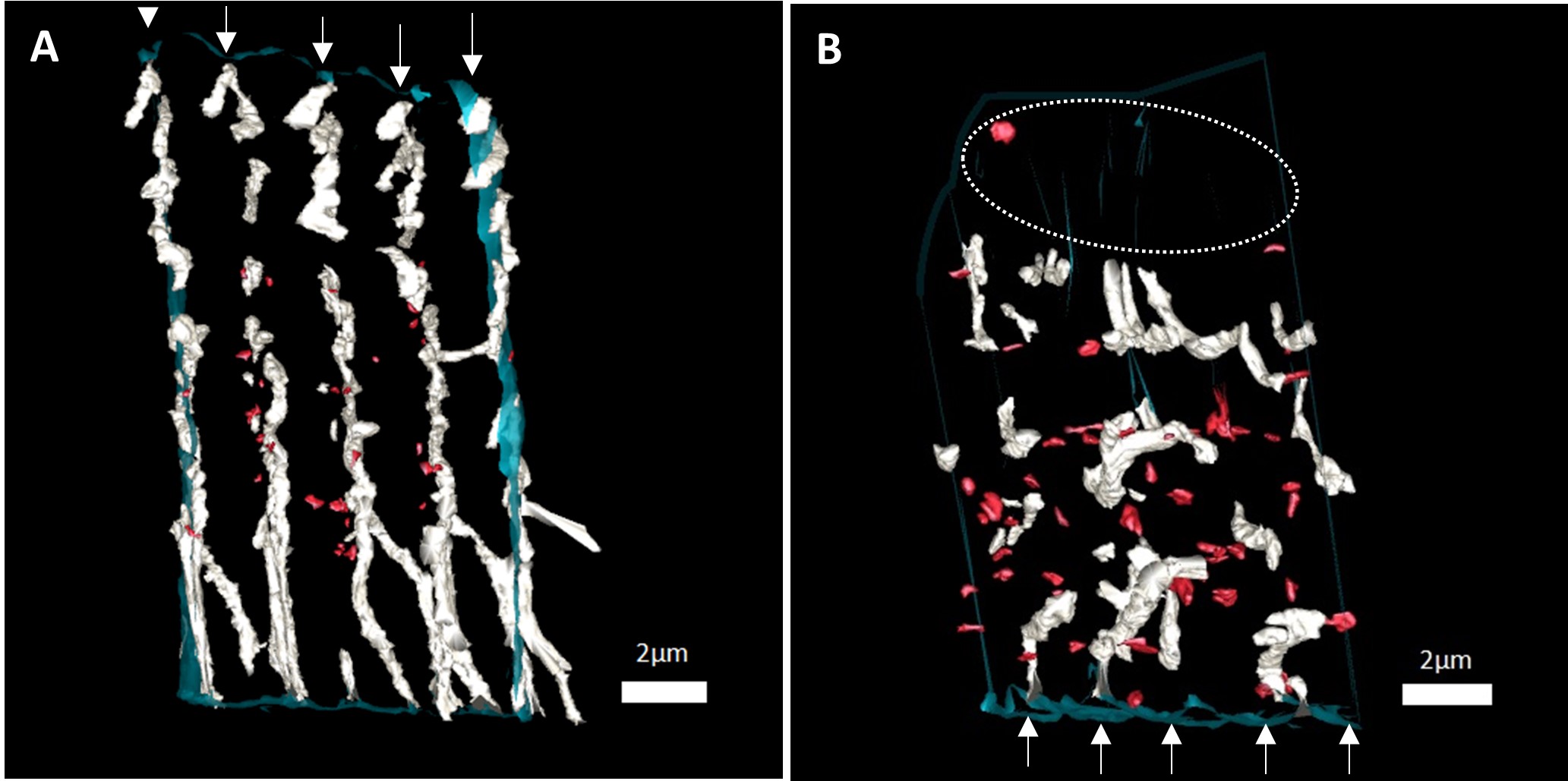
MI resulted in marked disorganisation of the t-tubule network (Fig 1). Quantitative analysis revealed that in comparison to the control myocardium, the MI border zone had a decreased t-tubule count (0.05 ± 0.004 tubule per μm3 in MI vs 0.07 ± 0.007 tubule per μm3 in control; p = 0.02) and showed t-tubule minor axis dilation (533 ± 30 nm vs 405 ± 22 nm; p = 0.02). While there was minimal disorganisation and loss of t-tubules in the MI remote region, we observed increased t-tubule length as a fraction of the cell diameter in the remote region post-MI (0.56 ± 0.04 vs 0.41 ± 0.04; p = 0.045). We suggest that this is likely a compensatory adaptation of the remote region in response to loss of t-tubules in the border region following MI. Importantly, we found a striking increase in t-tubule fragmentation in the MI border region (Fig 1B), a feature not observed in the remote region. These structural alterations in t-tubules are likely to manifest in disruptions to intracellular Ca2+ handling including slowed rise and decay of Ca2+ transients, spontaneous Ca2+ sparks and reduced amplitude of Ca2+ transients. Subsequently, this is likely to contribute to contractile dysfunction and arrhythmias following MI.
Figure 1: 3D renderings of the t-tubule network in healthy and MI border sheep cardiac myocytes. 3D renderings of the t-tubule network in a A) healthy cardiac myocyte and a B) MI border region cell, in longitudinal view, with the sarcolemma shown in blue, t-tubules in white, and fragments in red. Z-lines are indicated by white arrows. A) An ordered arrangement of t-tubules, perpendicular to the sarcolemma and regularly along z-lines. B) Disorganisation of t-tubules, t-tubule fragmentation, and regional t-tubule loss indicated by an ellipsoid. Scale bars= 2μm. Abbreviations: 3D, three-dimensional.
Conclusions
Our research extends the findings of previous studies on t-tubule structural changes in heart failure. This study highlights novel t-tubule remodelling features such as: fragmentation in the border region and increased length in the remote region, which may provide novel therapeutic drug targets for the management of cardiac dysfunction post-MI, and prevent deterioration of cardiac function in patients following MI. Future work will include exploration of the temporal evolution of t-tubule remodelling, the mechanisms underpinning t-tubule fragmentation and its association with functional consequences.
- References