Multi-modal chemical imaging of catalysts – from micro to nano scale resolution
- Abstract number
- 126
- Presentation Form
- Submitted Talk
- Corresponding Email
- [email protected]
- Session
- Stream 4: Diamond Light Source Session 2
- Authors
- Dorota Matras (2, 5), Antonios Vamvakeros (3, 1), Simon Jacques (3), Andrew Beale (1, 4, 3), Paul Quinn (2)
- Affiliations
-
1. Department of Chemistry, University College London
2. Diamond Light Source
3. Finden Ltd
4. Research Complex at Harwell
5. The Faraday Institution
- Keywords
chemical imaging, tomography, catalysis, diffraction, spectroscopy
- Abstract text
Summary
Chemical imaging techniques were applied to investigate the complex solid-state chemistry in catalytic systems used for methane conversion processes. The results, obtained with high resolution diffraction and spectroscopic imaging, improved our understanding of the catalyst structure-function relationships.
Introduction
Heterogenous functional materials, such as solid catalysts and battery materials typically possess a non-uniform 3D structure and are known to change with time under operating conditions. High resolution chemical imaging techqniues have the potential to provide a unique insight into the complex structure-function relationships. For example, the combination of computed tomography (CT) with techniques such as X-ray diffraction (XRD), X-ray fluorescence (XRF) and X-ray absorption near edge spectroscopy (XANES) enables for the extraction of local chemical and physical state information within the interiors of intact materials 1,2 . The obtained spatially-resolved signals can reveal information that would otherwise be lost in bulk measurements. We show here how such chemical imaging techniques have been applied to study catalytic materials at microfocus (I18) and nanofocus (I14) beamlines of the Diamond Light Source.
Materials and methods
1. Materials
The 2 wt.% La - 2 wt.% Mn – 1.6 wt.% Na – 3.1 wt.% W/SiO2 and the 2 wt.% Mn – 1.6 wt.% Na – 3.1 wt.% W/SiO2 catalysts were prepared by the sequential incipient wetness impregnation method. The 10% wt.% Ni – 0.2 wt.% Re/10 wt.% CeO2-ZrO2/Al2O3 catalyst was prepared by sequential impregnation method. All materials were kindly provided by the Boreskov Institute of Catalysis (BIC).
2. Methods
Combined μ-XRF-CT and μ-XRD-CT measurements were performed at beamline I18 of the Diamond Light Source using a 13 keV monochromatic X-ray beam focused to a spot size of 2.3 μm (V) × 3.5 μm (H). XRF spectra were collected with the Standard Vortex Si Drifts detector and the XRD patterns were collected with a Photonic Sciences CMOS-based X-ray imaging detector with exposure time of 350 ms per point. Catalyst particles were placed inside a quartz capillary (0.5 mm outer Ø and 0.4 mm inner Ø) supported by glass wool. Tomographic measurements were performed with 125 translation steps (with 5 μm step size) and 55 angular steps (with 3.5° step size) covering angular range from 0 to 189°. The reaction mixture (50 % of CH4/Ar and 20 % of O2/He) with the molar ratio of CH4/O2 equal 4 was introduced to reactor during the measurements, while the reactor was heated to 800 °C by an infrared heater. Every 2D diffraction image was converted to a 1D powder diffraction pattern after applying a 30% trimmed mean filter to suppress the single-crystal diffraction artefacts using in-house developed MATLAB scripts 2.
Combined 3D-XRF-CT and 3D-XRD-CT measurements were performed at beamline I14 of the Diamond Light Source using a 18 keV monochromatic X-ray beam focused to a spot size of ~ 50 nm (V) × 50 nm (H). XRF spectra were collected with the XRF SDD in the backscatter geometry and the XRD patterns were collected with the Excalibur detector in transmission geometry with exposure time of 200 ms per point. Catalyst particle was mounted on a standard tomography pin. Tomographic measurements were performed with 230 translation steps (with 800 nm step size), 37 vertical steps (with 800 nm step size) and 46 angular steps (with 4° step size) covering angular range from 0 to 180°.
Results and discussion
1. Na2WO4-Mn2O3/SiO2 catalyst for oxidative coupling of methane
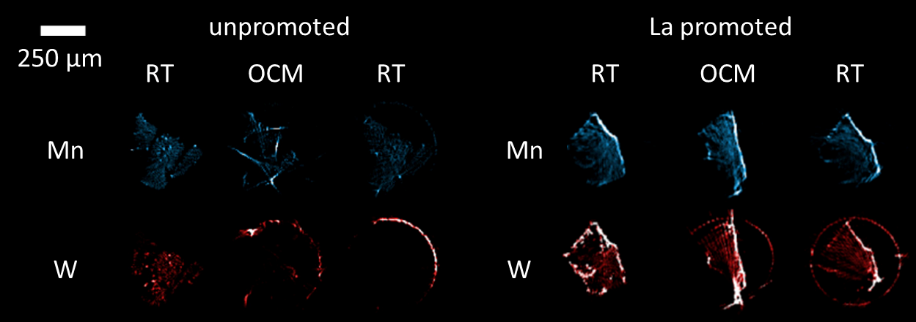
This study aimed to understand the role of La as a promoter in the Na2WO4-Mn2O3/SiO2 catalyst for the oxidative coupling of methane. Hence, both catalysts (i.e. the La promoted and the unpromoted) were measured under operating conditions with μ -XRF-CT and μ-XRD-CT. As revealed by XRF-CT (Figure 1), at high temperatures catalyst active W species become volatile and migrate from the catalyst particle towards the reactor vessel, a phenomenon that is observed to a lesser extent in the case of the La promoted catalyst. As shown with XRD-CT measurements, this could be explained by formation of NaLa(WO4)2 phase, which is more stable at high temperatures when compared to unpromoted Na2WO4 phase. In addition, the La promoter was found to act as a chemical promoter, enhancing the interaction between metallic species and support material through formation of La-Mn-Si-O phases 3.
Figure 1. Elemental maps of Mn and W of the unpromoted and La promoted Na2WO4-Mn2O3/SiO2 catalysts at room temperature, under OCM reaction and after OCM reaction at room temperature.
2. Ni-Re/CeO2-ZrO2/Al2O3 catalyst for methane reforming
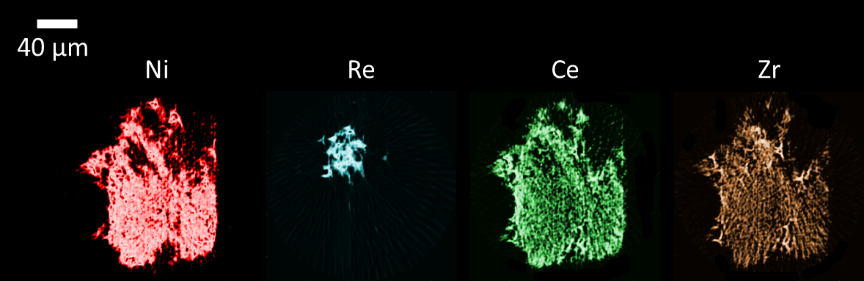
In this study we aimed to understand the role of the noble metal promoter (Re) and the redox promoters (CeO2-ZrO2) by investigating changes, induced during the reforming reaction, in the solid-state chemistry of a multi-component Ni-Re/CeO2-ZrO2/Al2O3 catalyst 4. With 3D-XRF-CT we observed a significant agglomeration of Re species at the surface of the catalyst particle. At the same time, Ce and Zr showed a similar distribution suggesting the formation of a mixed CeO2-ZrO2 redox material, with Ce species being also present in high concentration without Zr species in only a small volume of catalyst particle. These heterogeneities are bound to have an impact on how coke formation and sintering of metallic active species, will occur during the methane reforming reaction.
Figure 2. Elemental maps of Ni, Re, Ce and Zr of the Ni-Re/CeO2-ZrO2/Al2O3 catalyst at room temperature before reaction.
Conclusion
Chemical imaging methods allow us to investigate multi-component functional materials with micron and sub-micron resolution. Multi-modal imaging of catalyst particles with XRD-CT and XRF-CT provided a unique insight into the catalyst active site(s), the influence of each promoter on the catalyst performance and the reasons behind the catalyst deactivation.
- References
(1) Beale, A. M.; Jacques, S. D. M.; Weckhuysen, B. M. Chemical Imaging of Catalytic Solids with Synchrotron Radiation. Chem. Soc. Rev. 2010, 39 (12), 4656–4672. https://doi.org/10.1039/C0CS00089B.
(2) Matras, D.; Pritchard, J.; Vamvakeros, A.; Jacques, S. D. M.; Beale, A. M. Tomography in Catalyst Design. In Heterogeneous Catalysts; John Wiley & Sons, Ltd, 2021; pp 263–278. https://doi.org/10.1002/9783527813599.ch15.
(3) Vamvakeros, A.; Jacques, S. D. M.; Di Michiel, M.; Middelkoop, V.; Egan, C. K.; Cernik, R. J.; Beale, A. M. Removing Multiple Outliers and Single-Crystal Artefacts from X-Ray Diffraction Computed Tomography Data. J. Appl. Crystallogr. 2015, 48 (6), 1943–1955. https://doi.org/10.1107/S1600576715020701.
(4) Vamvakeros, A.; Matras, D.; Jacques, S. D. M.; di Michiel, M.; Price, S. W. T.; Senecal, P.; Aran, M. A.; Middelkoop, V.; Stenning, G. B. G.; Mosselmans, J. F. W.; Ismagilov, I. Z.; Beale, A. M. Real-Time Multi-Length Scale Chemical Tomography of Fixed Bed Reactors during the Oxidative Coupling of Methane Reaction. J. Catal. 2020, 386, 39–52. https://doi.org/10.1016/j.jcat.2020.03.027.
(5) Matus, E. V.; Ismagilov, I. Z.; Yashnik, S. A.; Ushakov, V. A.; Prosvirin, I. P.; Kerzhentsev, M. A.; Ismagilov, Z. R. Hydrogen Production through Autothermal Reforming of CH4: Efficiency and Action Mode of Noble (M = Pt, Pd) and Non-Noble (M = Re, Mo, Sn) Metal Additives in the Composition of Ni-M/Ce0.5Zr0.5O2/Al2O3 Catalysts. Int. J. Hydrog. Energy 2020, 45 (58), 33352–33369. https://doi.org/10.1016/j.ijhydene.2020.09.011.