Mesoscopic imaging of excised pediatric palatine tonsils confirms the presence of bacterial infection beyond the surface
- Abstract number
- 149
- Presentation Form
- Submitted Talk
- Corresponding Email
- [email protected]
- Session
- Stream 5: Up Close with the Enemy: Imaging Pathogen-host Dynamics
- Authors
- Ms Megan Clapperton (2), Professor Gail McConnell (2), Ms Catriona Douglas (1)
- Affiliations
-
1. Royal Hospital for Children
2. University of Strathclyde
- Keywords
Life sciences; light microscopy; disease; tissue imaging.
- Abstract text
Recurring tonsillitis in the paediatric age group is one of the most common problems presented to GPs in the UK, with an annual reported incidence of 37 per 1000 population in the UK [1]. Recurring tonsillitis can have detrimental effects on children’s quality of life with repeated school absences and the trauma that can be involved with hospital visits. Often, antibiotic treatment of the infection does not suffice and if a patient has seven or more cases of tonsillitis per year the complete removal of the tonsils is recommended [2]. This operation is not without risk and 20% of tonsillectomy patients end up being readmitted within 2 weeks of surgery due to pain or complications.
Studies into tonsillitis infections have been performed previously in a set of 15 tonsillectomy specimens by Chloe and Faddis, this showed the presence of gram-positive and gram-negative bacteria within the tonsil crypts [3]. These present the ultrastructural appearance of a biofilm matrix. Biofilms are communities of bacteria which are embedded within an extracellular polymeric substances and are well known to be involved in the chronicity of infections as well as possessing a resistance to antibiotic treatments [4].
We report the first demonstration of mesoscopic imaging of bacterial infection of the palatine tonsil with use of the Mesolens. The Mesolens, developed at the University of Strathclyde, offers the unique combination of a low magnification (4x) and high numerical aperture (0.47) lens which has an imaging area of 6 mm x 6 mm. The Mesolens can resolve features as small as 700nm laterally and 7um axially [5], hence thousands of individual bacteria are clearly resolved in a single image, and therefore can provide a more representative view of the specimen. If a low level bacterial infection is present it may be missed using an ordinary light microscope, but the field of view of the Mesolens means that the probability of detecting a few bacteria in the specimen is greatly increased.
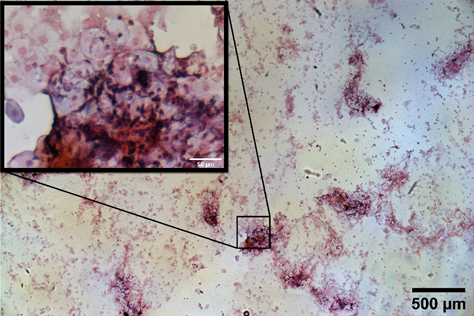
Samples were collected via a swab from the surface of unfixed palatine tonsil tissue acquired via tonsillectomy at the NHS Royal Hospital for Children, Glasgow, which had been transported and stored in PBS for 2 hours before processing. Specimens were prepared using a standard Gram stain protocol with Crystal Violet dye staining gram positive cells blue / purple, and the counter stain Safranin staining gram negative cells red / pink . Figure 1 shows a brightfield Mesolens image of mammalian tissue from the tonsil as well as bacteria, with thousands of bacterial cells clearly resolved.
Figure 1 Brightfield colour image of Gram-stained specimen imaged with the Mesolens objective lens. A region of interest is chosen and shown in the inset, revealing the tonsil cells in pink and bacteria in purple.
Using the Mesolens to study the infection of palatine tonsils offers a distinct advantage over conventional objective lenses. With a much increased field of view and a sub-cellular resolution throughout, it is possible to understand the prevalence of bacteria in much larger specimens than can normally be studied with a light microscope, and any infection, however small, has a greater chance of being detected using the Mesolens.
- References
[1] Douglas CM, Altmyer U, Cottom L, Young D, Redding P, Clark LJ. A 20-year observational cohort of a 5 million patient population-Tonsillectomy rates in the context of two national policy changes. Clin Otolaryngol. 2019;44(1):7
[2] .SIGN. Management of sore throat and indication of tonsillectomy. SIGN 117. 2010; http://www.sign.ac.uk/guidelines/fulltext/117/.
[3] Chole, R.A. and Faddis, B.T., 2003. Anatomical evidence of microbial biofilms in tonsillar tissues: a possible mechanism to explain chronicity. Archives of Otolaryngology–Head & Neck Surgery, 129(6), pp.634-636.
[4] Römling, U. and Balsalobre, C., 2012. Biofilm infections, their resilience to therapy and innovative treatment strategies. Journal of internal medicine, 272(6), pp.541-561.
[5] McConnell, G. and Amos, W.B., 2018. Application of the Mesolens for subcellular resolution imaging of intact larval and whole adult Drosophila. Journal of microscopy, 270(2), pp.252-258.