High resolution imaging and spectroscopy of interfaces in solid-state Li-ion batteries
- Abstract number
- 35
- Presentation Form
- Poster
- Corresponding Email
- [email protected]
- Session
- Poster Session 3
- Authors
- Ms. Ruomu Zhang (1), Dr. Weixin Song (1, 2), Prof. Peter Bruce (1, 2, 3), Prof. Peter Nellist (1)
- Affiliations
-
1. University of Oxford
2. The Faraday Institution
3. The Henry Royce Institute
- Keywords
Solid-state Li ion batteries, Scanning transmission electron microscopy, Solid-state electrolyte/cathode interface
- Abstract text
Summary: Solid-state electrolyte (SSE) Argyrodite Li6PS5Cl and Ni-rich cathode material LiNi0.6Mn0.2Co0.2O (NMC622) have great potential in future battery technologies benefitting from high energy density and improved operational safety. However, they currently suffer from rapid capacity fading issues due to the side reaction at the SSE/cathode interface. In this work, we use simultaneous annular dark field (ADF)/energy dispersive X-ray spectroscopy (EDX)/electron energy loss spectroscopy (EELS) in scanning transmission electron microscopy (STEM) to identify the changes of microstructure, chemical homogeneity and oxidation states at the NMC622/Argyrodite interface upon spontaneous reaction. Compared to the pristine NMC622, no obvious changes are observed in structure or chemical homogeneity, while the Ni content in NMC622 has been partially oxidised by Argyrodite. HRTEM images of Argyrodite reveal that amorphisation is the primary effect of beam damage which needs to be differentiated from cycling decomposition.
Solid-state lithium-ion batteries (SSLIBs) are promising next-generation energy storage systems, owing to their significantly improved safety while still maintaining high energy density compared to conventional liquid electrolyte system lithium-ion batteries. [1] However, the capacity fading and open-circuit voltage hysteresis issues need to be resolved before commercialization. Previous studies have suggested that these losses are closely related to the side reactions at the SSE/cathode interface [2]. To gain insights into the origin of degradation and failure mechanisms in SSLIBs, the structure and composition changes of SSE/cathode interphase will be comprehensively studied in this work.
STEM is one of the most widely used atomic-scale characterisation technique. Atomic position imaging, elemental mapping and electronic structure information can be simultaneously acquired with ADF, EDX and EELS detectors. In this work, we applied ADF/EDX/EELS to study the interface between cathode material NMC622 and SSE Argyrodite Li6PS5Cl before/after cycling. Moreover, to distinguish the decomposition changes from beam damage and cycling, the pristine form of argyrodite is examined in TEM for diffraction pattern evolution upon electron beam exposure.
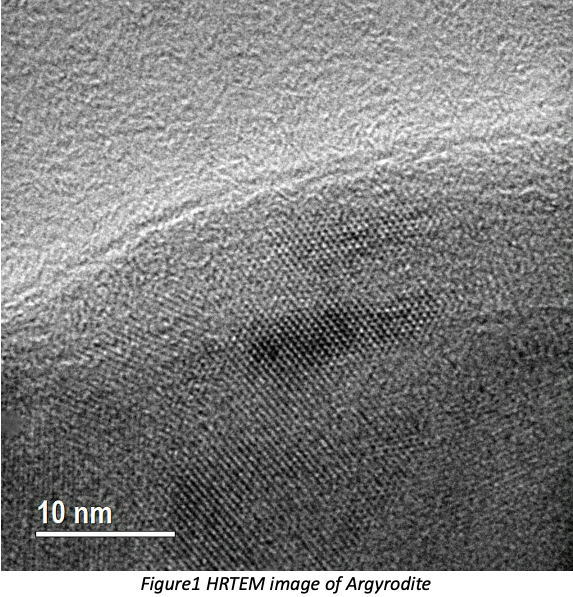
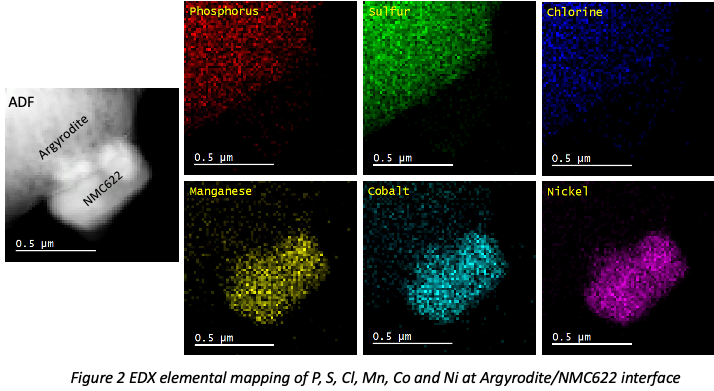
Atomic-resolution HRTEM images of Argyrodite (Fig.1) have been successfully acquired and the crystallites have grain size of 20-30nm while an amorphous phase is also present. In the interface study, EDX elemental mapping (Fig.2) identifies an interface between NMC622 and Argyrodite. ADF images provide structural information, while EEL spectra reveal the oxidation state at high spatial resolution simultaneously. The oxidation state is determined by white-line intensity ratio L3/L2, and L3/L2 mapping will be discussed in the presentation.
It is observed in TEM that the primary effect of beam damage is amorphisation. Compared to the pristine structure, NMC622 is partially oxidised when in contact with Argyrodite due to the spontaneous reaction at SSE/cathode interface. With the comprehensive analysis of the microstructure and chemistry of SSE/cathode interphase by using ADF/EDX/EELS in STEM, our work can provide a guide for future materials selection in SSLIBs by correlating the microstructure and composition with electrochemical performance.
- References
[1] N. Naoki et al. Mater. Today 18.5 (2015): 252-264
[2] A. Jeremie et al. Chem. Mater. 29.9 (2017): 3883-3890
[3] The authors acknowledge use of characterization facilities within the David Cockayne Centre for Electron Microscopy, Department of Materials, University of Oxford and in particular the Faraday Institution (FIRG007, FIRG008), the EPSRC (EP/K040375/1 “South of England Analytical Electron Microscope”) and additional instrument provision from the Henry Royce Institute (Grant reference EP/R010145/1).