Gold Oxide Formation on TiO2/Au(111) Model Catalysts
- Abstract number
- 252
- Presentation Form
- Submitted Talk
- Corresponding Email
- [email protected]
- Session
- Stream 3: Operando Microscopy
- Authors
- Sabine Wenzel (1), Irene Groot (1)
- Affiliations
-
1. Leiden Institute of Chemistry
- Keywords
- Abstract text
Hydrogen produced from methanol has to be cleaned from traces of CO for its use in fuel cells [1]. Conventional CO oxidation catalysts such as platinum and palladium oxidize hydrogen as well and are thus not suitable. Alternative gold-based catalysts have been shown to selectively oxidize CO at low temperatures [2]. There is ample evidence for strong interactions between gold and typically used supports such as TiO2 [3]. However, the exact oxidation state of the active phase of gold remains under debate [4,5]. Additionally, there is evidence that water plays a role in the activity and might make the oxide support unnecessary [6].
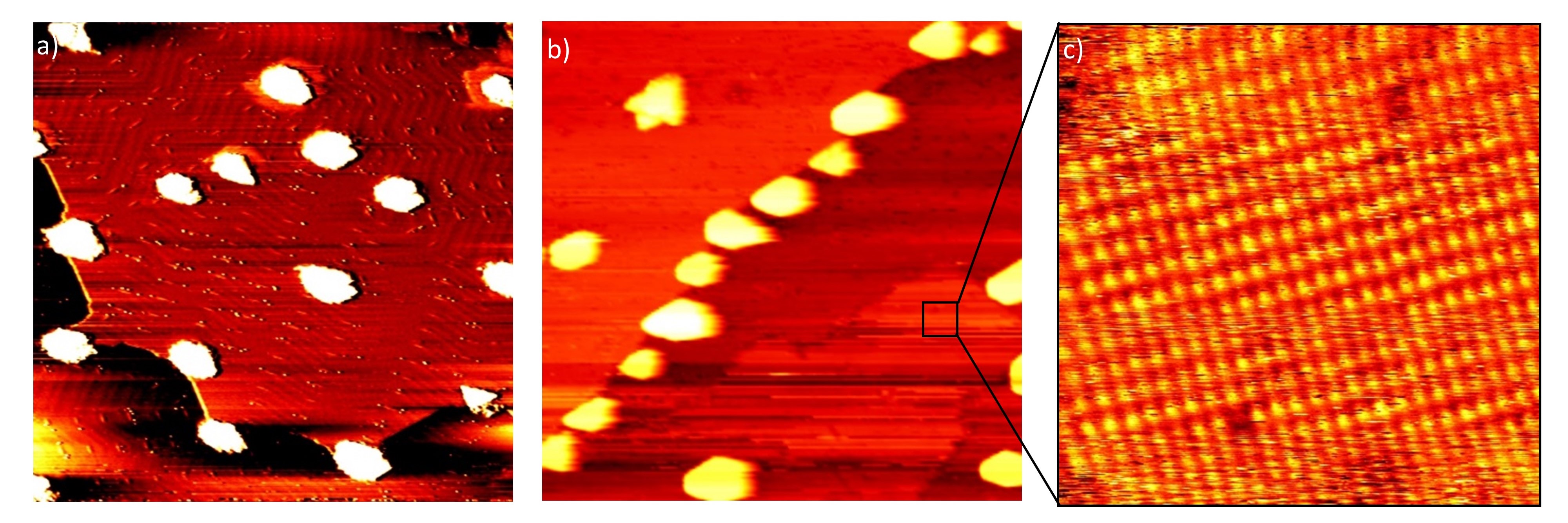
Our set-up [7] allows for the controlled preparation and characterization of model catalyst surfaces in ultra-high vacuum combined with scanning tunneling microscopy at atmospheric pressures and elevated temperatures. A TiO2/Au(111) model catalyst was prepared via physical vapor deposition and exposed to CO oxidation reaction conditions. We present evidence for the formation of a surface gold oxide in this environment as can be seen in Figure 1. We will discuss the role that the titania nanoparticles, contaminants on the gold substrate, and the water background in the gas mixture play in the oxidation of the gold substrate. Our findings suggest that transfer of atomic oxygen from the titania nanoparticles to the gold substrate does not occur.
Fig. 1: a) 120 nm x 120 nm image of the as-prepared TiO2/Au(111) model catalyst. b) 120 nm x 120 nm image of the same surface after exposure to 1 bar of 4 O2 + 1 CO for one hour showing the surface gold oxide. c) 10 nm x 10 nm zoom of the marked region in b) showing the squared unit cell of the surface gold oxide.
- References
[1] Dhar et al., J. Electrochem. Soc. 1987, 134, 12, 3021.
[2] Haruta, The Chemical Record 2003, 3, 75–87.
[3] Palomina et al., ACS Sustainable Chem. Eng. 2017, 5, 10783.
[4] Min et al., Chem. Rev. 2007, 107, 2709.
[5] Klyushin et al., ACS Catal. 2016 6 , 3372.
[6] Kettemann et al., ACS Catal. 2017, 7, 8247.
[7] Herbschleb et al., Rev. Sci. Instrum. 2014, 85, 083703.