Engineered Point Spread Functions Enable Axial Super-Resolution in Single Molecule Imaging and Single Particle Tracking
- Abstract number
- 333
- Presentation Form
- Poster
- Corresponding Email
- [email protected]
- Session
- Poster Session 3
- Authors
- Anurag Agarwal (1), Warren Colomb (1), Scott Gaumer (1), Anjul Loiacono (1), Leslie Kimerling (1)
- Affiliations
-
1. Double Helix Optics
- Keywords
Single Molecule Microscopy, Super-resolution, 3D, Point Spread Function, Double Helix PSF, 3D Imaging, Superresolution, Localization, Tracking, Spatial
- Abstract text
Single molecule localization microscopy (SMLM) is a family of imaging techniques that enables nanometer scale visualization by localizing sparse sets of photo-switchable/photo-activatable molecules over many frames [1], [2]. Combining the localization data over 1000’s of frames, acquired with this technique, results in a single “super-resolution image”. Techniques that fall within the SMLM family include STORM, PALM, DNA-PAINT, etc. While SMLM techniques offer unprecedented precision of 10-20nm in the lateral dimension, they lack any axial (z) resolution, especially near the focus.
To begin to try and achieve axial resolution, the introduction of an astigmatic lens to shape the point spread function (PSF) in a controlled manner, has been implemented [3]. A limitation to this approach is that it has a limited depth capability of only 500 – 700nm. SMLM studies, however, are often performed in cells and bacteria that are thicker than this.
Our approach to this limitation involves engineering the point spread function using a phase-only mask to match the depth requirements of the sample under observation. Our Double Helix Point Spread Function (DH-PSF) is one such type of PSF that will extend the depth of field and provide high 3D precision for SMLM applications[4], [5].
DH-PSF is a family of engineered PSFs that modify the microscope’s Airy Disc PSF pattern such that the image of each emitter appears as two well-separated lobes. As an emitter moves in the axial direction, unlike the blurring of the Airy Disc, the two Double Helix lobes stay in focus and rotate around a center position, as a function of the axial position. The rotational angle of the lobes encodes the axial position and the center between the lobes represents the lateral location. This approach of using a Double Helix PSF has been shown to provide higher precision than other 3D techniques for a given depth of field. DH-PSF techniques have been demonstrated in not only 3D SMLM, but also 3D particle tracking[6], light sheet applications[7], and whole cell single molecule imaging[8].
To demonstrate the application of the DH-PSF, microtubules in a mouse embryonic fibroblast (MEF) were imaged with the SPINDLETM and 3D super-resolution images were reconstructed. Additionally, we compared Double Helix 3D super-resolution reconstructions to simulated 2D reconstructions. The microtubules were labeled with AlexaFluor 647 and imaged using a 1.49 NA/100x objective. The phase mask was implemented in a Double Helix SPINDLE module that sits between the microscope and the camera [9]. The sample was imaged continuously with a 640 nm laser under oblique illumination with an exposure time of 30 ms. The resulting images were analyzed using the Double Helix 3DTRAX™ software. The lateral and axial precision values were calculated by using the Cramer-Rao Lower Bound (CRLB) [5] for the experimental setup and based on the signal and background of each localized emitter.
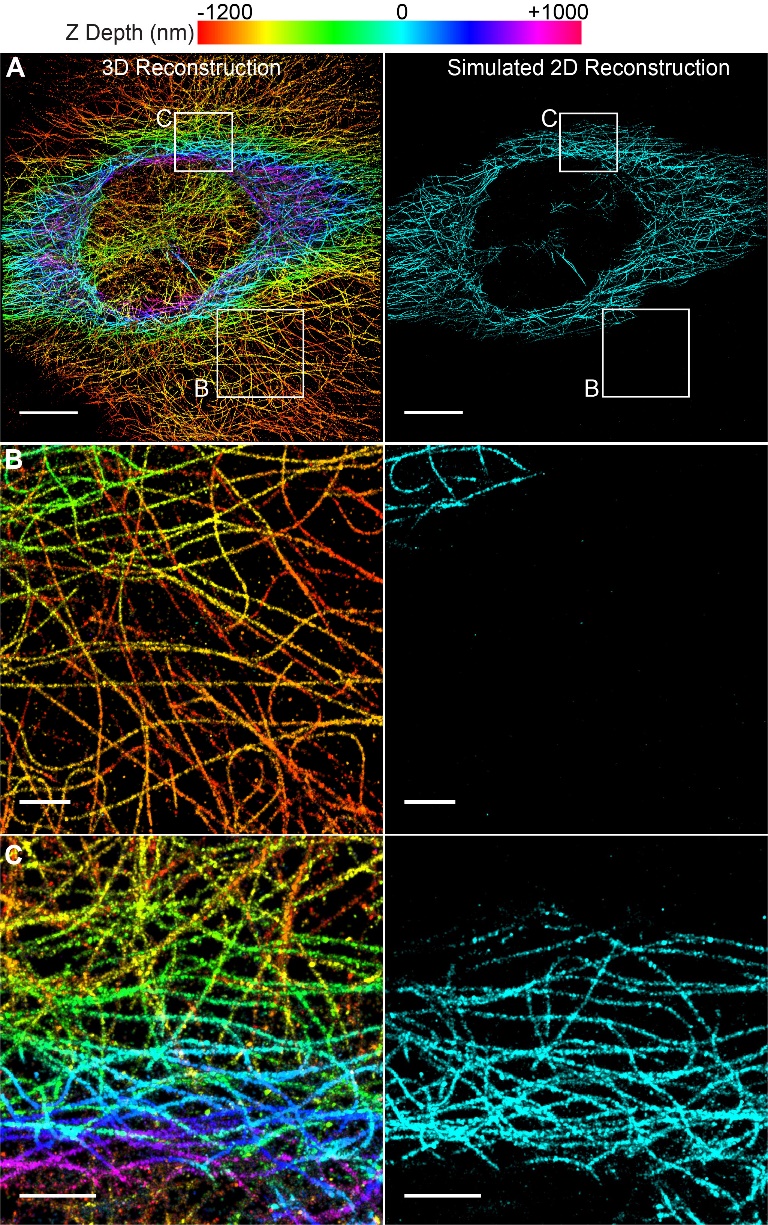
The resulting image captured a depth of 2.2 μm (Fig 1a). To compare the performance to 2D super resolution imaging, we generated simulated 2D images from the 3D data. We based our simulated reconstruction on typical values reported for 2D super-resolution (approximately 1 micron of depth). These simulated data were reconstructed using only emitters in the center one micron (-500 nm to +500 nm) of the axial (z) dimension of the dataset. Additionally, all z depth information is lost in the 2D image, therefore it is displayed in only one color (Fig 1A-C, right panels).
When compared to the simulated 2D data, the Double Helix 3D images captured over twice the depth with precise z-position information (Fig 1). As can be seen in the figure, microtubules at the bottom of the cell, colored orange to yellow, are completely lost in the 2D image (Fig 1B). In contrast, the extended depth of field of the Double Helix mask enabled imaging and reconstruction of these microtubules. Additionally, the 3D Double Helix reconstruction allowed easy distinction of microtubules at different z-depths of the cell (Fig 1C).
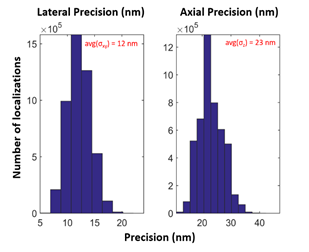
In addition to the extended depth of field, Double Helix PSF Engineering enables collection of high precision localization data in all three dimensions (x, y, and z). To demonstrate this precision, the standard deviation (sigma) of the x, y, z precision for the 3D reconstruction shown in Fig.1 are graphed in Fig. 2. For this dataset, the average sigma values for lateral (x-y), and axial (z) precision were 12 nm and 23 nm, respectively. In conclusion, the SPINDLETM enabled generation of a high-quality super-resolution image with extended z range and high lateral and axial precision.
Methods: Cell Culture and Immunofluorescence Staining: Mouse embryonic fibroblasts (MEF) from ATCC (SCRC-1008) were fixed in 4% paraformaldehyde and 0.1% glutaraldehyde, permeabilized with 0.1% Triton-X100 and blocked overnight in 10% serum. The samples were incubated with an AlexaFluor 647 conjugated primary α-tubulin antibody (Novus Biologicals) for 2 hours. The samples were then post-fixed in 4% PFA and 0.1% GA. Image Analysis and Rendering: Individual fluorophores were localized using the 3DTRAX™ software which returns the 3D (x, y, z) locations, intensities, and dimensional precisions (σx, σy, σz) of the fluorophores. These data were then drift corrected using cross correlations (number of bins = 10), filtered by intensity and density and then plotted with a normalized Gaussian reconstruction.
Figure 1| 3D Super-Resolution Reconstruction of Microtubules Imaged with SPINDLE™. (A) 3D with Double Helix and simulated 2D reconstructions showing z-depth encoded in color (scale at top). The simulated 2D reconstruction shows 1 μm of z-depth (-500 to +500 nm) and does not contain any axial localization information. Scale bar is 10 μm. (B) Enlarged region of reconstructions in A, showing extended depth of the Double Helix. Scale bar is 2 μm (C) Enlarged region of reconstructions in A, showing easy depth distinction in DH-3D image. Scale bar is 2 μm.
Figure 2| Precision values calculated by 3DTRAX™ software for localizations in Fig. 1 reconstruction. The average sigma (σ) values for this dataset were σxy = 12 nm and σz = 23 nm.
- References
[1] M. J. Rust, M. Bates, and X. Zhuang, “Sub-diffraction-limit imaging by stochastic optical reconstruction microscopy (STORM).,” Nat. Methods, vol. 3, no. 10, pp. 793–5, Oct. 2006.
[2] E. Betzig et al., “Imaging intracellular fluorescent proteins at nanometer resolution.,” Science, vol. 313, no. 5793, pp. 1642–5, Sep. 2006.
[3] B. Huang, W. Wang, M. Bates, and X. Zhuang, “Three-dimensional super-resolution imaging by stochastic optical reconstruction microscopy.,” Science, vol. 319, no. 5864, pp. 810–3, Feb. 2008.
[4] S. R. P. Pavani et al., “Three-dimensional, single-molecule fluorescence imaging beyond the diffraction limit by using a double-helix point spread function,” Proc. Natl. Acad. Sci., vol. 106, no. 9, pp. 2995–2999, Mar. 2009.
[5] S. R. P. Pavani and R. Piestun, “High-efficiency rotating point spread functions,” Opt. Express, vol. 16, no. 5, p. 3484, Mar. 2008.
[6] D. Wang, H. Wu, L. Liu, J. Chen, and D. K. Schwartz, “Diffusive Escape of a Nanoparticle from a Porous Cavity,” Phys. Rev. Lett., vol. 123, no. 11, p. 118002, Sep. 2019.
[7] A.-K. Gustavsson, P. N. Petrov, M. Y. Lee, Y. Shechtman, and W. E. Moerner, “3D single-molecule super-resolution microscopy with a tilted light sheet,” Nat. Commun. 2018 91, vol. 9, no. 1, p. 123, Jan. 2018.
[8] A. R. Carr et al., “Three-Dimensional Super-Resolution in Eukaryotic Cells Using the Double-Helix Point Spread Function.,” Biophys. J., vol. 112, no. 7, pp. 1444–1454, Apr. 2017.
[9] S. Jain, J. R. Wheeler, R. W. Walters, A. Agrawal, A. Barsic, and R. Parker, “ATPase-Modulated Stress Granules Contain a Diverse Proteome and Substructure,” Cell, vol. 164, no. 3, pp. 487–498, 2016.