Correlative Soft Tissue Synchrotron Microtomography: Sample Preparation, Imaging, Reconstruction and Segmentation Methods
- Abstract number
- 177
- Presentation Form
- Poster Flash Talk + Poster
- DOI
- 10.22443/rms.mmc2021.177
- Corresponding Email
- [email protected]
- Session
- Stream 4: Diamond Light Source Session 2
- Authors
- Andrew J Bodey (1), Merrick C Strotton (3), Win Tun (1), Alexey V Buzmakov (2), Victoria Gulimova (4), Kazimir Wanelik (1), Michelle C Darrow (1), Elizabeth J Bradbury (3), Christoph Rau (1)
- Affiliations
-
1. Diamond Light Source
2. FSRC "Crystallography and Photonics" RAS
3. King’s College London
4. Research Institute of Human Morphology, Ministry of Science and Higher Education RF
- Keywords
soft tissue, central nervous system, spinal cord, synchrotron, tomography, CT, reconstruction, machine learning, segmentation
- Abstract text
Introduction
Thousands of soft tissue microtomography experiments are conducted at synchrotrons around the world each year, and the quality of results varies widely. Soft biological tissues pose a particular challenge for synchrotron tomography, owing to poor contrast and susceptibility to deformation and beam damage artefacts. The rationale behind the choice of sample preparation methods, imaging parameters and reconstruction strategy is not always reported in articles, and so we conducted a systematic investigation of these aspects of experimental design for central nervous system samples. Computational segmentation can be particularly challenging for soft-tissue tomograms, and so we demonstrate the use of supervoxel-based machine-learning segmentation of our data.
Sample Embedding
Careful sample preparation is crucial for effective imaging of soft tissues. A sample which is optimally oriented, stained throughout and embedded so as to provide rigidity and avoid streak artefacts will deliver excellent results. In order to achieve good inline phase contrast for weakly-absorbing soft tissues, one invariably risks strong fringes at the interface between the embedding medium and air, leading to streak artefacts. The medium will therefore ideally sit outside the field of view, but not by so much that it causes excessive beam absorption.
Following fixation, spinal cords were placed in a custom-made mould. Cords were balanced at either end on lips to ensure they were surrounded on all sides by an appropriate thickness of medium.
Samples must be stable on the length scale of the features one hopes to resolve. Soft tissue samples are often highly flexible, and thus benefit from embedding. We trialed various embedding media, and found that only paraffin and resin conferred good stability. Although paraffin is more prone to cracking than resin, it offers the advantage of reversibility; paraffin was removed after tomography so that other imaging techniques could be used. Paraffin cracking was avoided by hardening the beam and reducing flux [1].
Contrast
The poor contrast of soft tissues can make the visualisation and segmentation of features difficult. Both chemical and optical methods can boost contrast, and some combination of both can be advantageous. We trialed a variety of stains and found that iodine gave the best penetration and contrast – particularly for the central grey matter. We enhanced this chemical contrast with in-line phase contrast, the extent of which is controlled by the propagation distance between sample and detector. Fresnel fringes increase in amplitude and width as propagation distance is increased. We chose 160mm, as it provided good contrast but avoided the excessive blurring which results from wide fringes.
Signal:Noise
Full angular sampling requires π/2 × object radius projection images (~4000 for our experiments). Beyond this, the use of additional images is a convenient means of increasing dose and thereby boosting signal:noise. To determine the optimal number, we performed a 24,001-projection scan and reconstructed from subsets of this dataset. 6,000 projections was found to bring signal:noise reasonably close to saturation without taking so long as to be prohibitive to a beamtime’s efficiency.
Artefact Correction
Various imperfections in an imaging system can lead to reconstruction artefacts. Data were reconstructed with Savu [2], a modular pipeline, which incorporates plugins to correct for various artefacts including zingers (which result from stray X-rays hitting the detector chip directly) and optical distortions in the imaging system [3]. Correcting for distortions can make significant improvements to data accuracy, and thereby the true resolution of data (Fig. 1).
Figure 1. Tomographic slice generated (a) without and (b) with optical distortion correction.
Segmentation: Supervoxels and Machine Learning
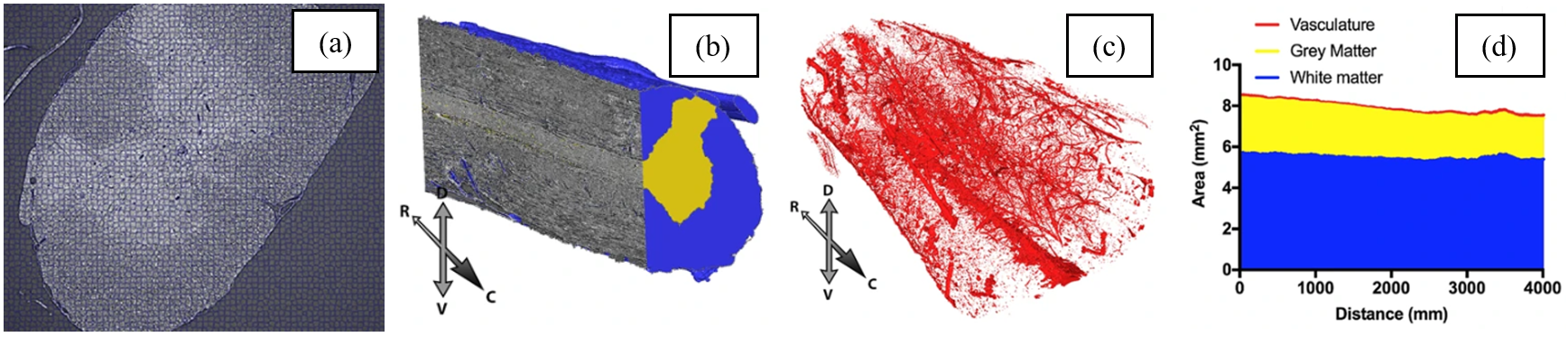
Computational segmentation of soft-tissue tomograms using traditional methods can be difficult. Humans, by contrast, are excellent at pattern recognition. This fact can be harnessed to make use of machine-learning software in which humans train algorithms to recognise patterns. Supervoxels – groups of similar, adjacent voxels – were used to speed up the process (Fig 2a). SuRVoS [4] was used for complete segmentation of grey and white matter and vasculature (Fig. 2b,c). Area measurements were taken along the length of the spinal cord (Fig. 2d). Such measurements could be useful for assessing changes in a variety of pathologies, such as spinal cord or brain injuries [5].
Figure 2. (a) Supervoxels. (b) Segmented grey and white matter. (c) Segmented vasculature. (d) Rostro-caudal measurements.
Correlative Imaging: Post-Tomography Histology
Histology can add molecular specificity to structures identified in 3D via tomography. After tomography, iodine was removed from the samples, which were then re-embedded in wax for sectioning and histology. This included the pan-neuronal marker NeuN, and generic tissue stains such as haematoxylin and eosin which stains cell nuclei (Fig. 3).
Figure 3. Histological images can be aligned to tomograms, with remarkable consistency of features. (a) Haematoxylin and eosin staining of 7μm-thick transverse tissue section. (b) Equivalent ‘6.4μm’ tomographic slice.
Tomography of Unstained Central Nervous System Tissues
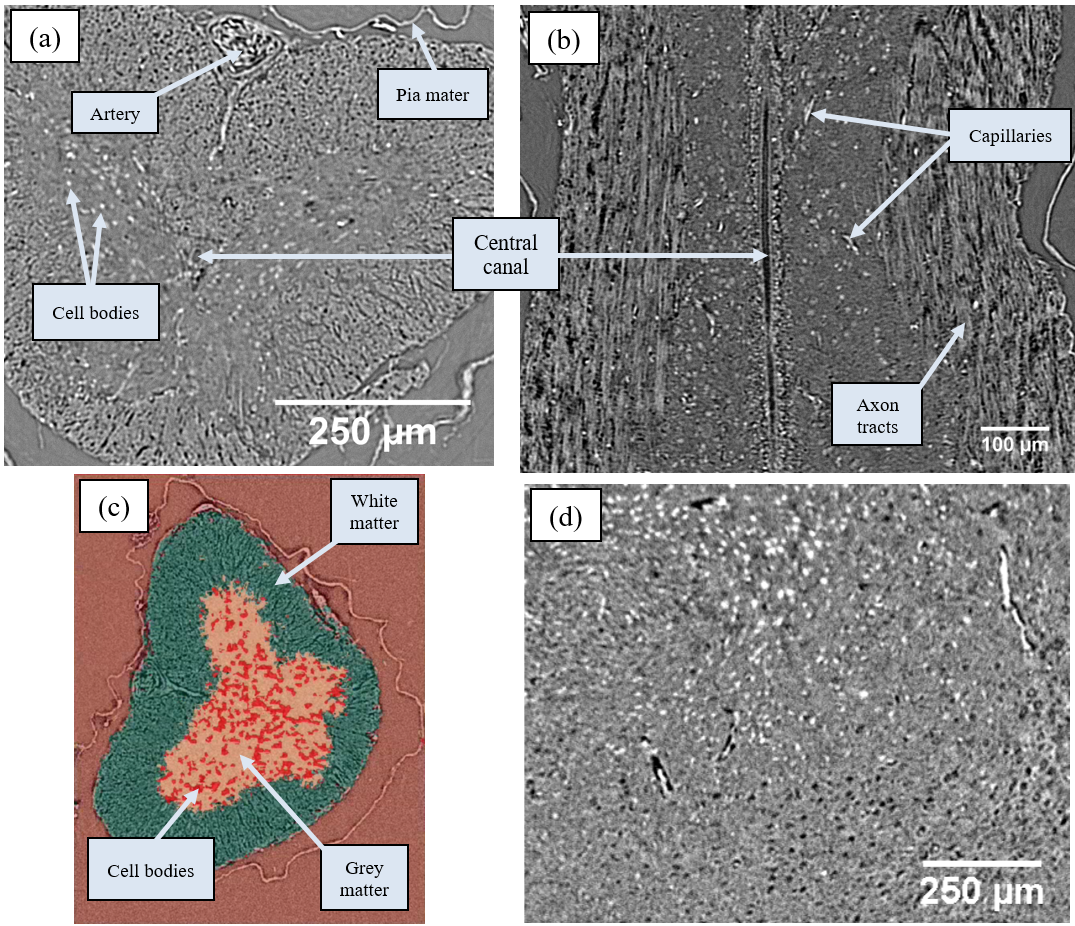
Good contrast – albeit via Fresnel fringes – can be achieved with unstained soft tissues via inline phase contrast alone. Spinal cords were sourced either from thick-toed geckos that had spent 30 days in space onboard unmanned spacecraft Bion-M1 (2013, Russia) – or a control group – as part of a blind study into the effects of spaceflight on animal tissues. Fixing and paraffin embedding conferred excellent stability to spinal cords and also whole mouse brains, and cracking could be avoided with suitable beam filtration. Various features could be clearly resolved (Fig. 4a,b,d). Cell bodies, grey matter and white matter were segmented from the spinal cords with SuRVoS (Fig. 4c), and these were used for rostro-caudal area measurements.
Figure 4. Unstained central nervous system tissues. (a) Transverse and (b) longitudinal sections of gecko spinal cord. (c) Segmentation of grey and white matter, and cell bodies. (d) Mouse brain tissue.
Acknowledgments
Tomographic imaging, reconstructions and analyses were conducted at I13-2 of Diamond Light Source, UK and its associated data beamline [6] (MT12538, MT14907 and MG23866). We thank A Maltsev and Е Shevtsova for preparing the mouse brain samples. The work was supported by King’s Bioscience Institute, Guy’s & St Thomas’ Charity Prize PhD Programme and MRC UK (G1002055).
Andrew Bodey and Merrick Strotton contributed equally to this project.
- References
1. Strotton MC & Bodey AJ (joint first authors), Wanelik K, Darrow MC, Medina E, Hobbs C, Rau C, Bradbury EJ. Scientific Reports. 8 (2018).
2. Atwood RC, Bodey AJ, Price SW, Basham M, Drakopoulos M. Philosophical Transactions of the Royal Society A: Mathematical, Physical and Engineering Sciences. 373 (2015).
3. Vo NT, Atwood RC, Drakopoulos M. Optics Express. 23 (2015).
4. Luengo I, Darrow MC, Spink MC, Sun Y, Dai W, He CY, Chiu W, Pridmore T, Ashton AW, Duke EMH, Basham M, French AP. 198 (2017).
5. Strotton MC, Bodey AJ, Wanelik K, Hobbs C, Rau C, Bradbury EJ. Experimental Neurology. 336 (2020).
6. Bodey AJ, Rau C. Journal of Physics: Conference Series 2017. 849 (2017). IOP Publishing.