Correction of multiple-blinking artefacts in photoactivated localisation microscopy
- Abstract number
- 162
- Presentation Form
- Submitted Talk
- DOI
- 10.22443/rms.mmc2021.162
- Corresponding Email
- [email protected]
- Session
- Stream 6 (Frontiers): Development and Applications in Super Resolution Microscopy
- Authors
- Louis G Jensen (2), Tjun Yee Hoh (3), David J Williamson (6), Juliette Griffié (5), Daniel Sage (1), Patrick Rubin-Delanchy (3), Dylan M Owen (4)
- Affiliations
-
1. Biomedical Imaging Group, School of Engineering, EPFL
2. Department of Mathematics, Aarhus University
3. Institute for Statistical Science, School of Mathematics, University of Bristol
4. Institute of Immunology and Immunotherapy, School of Mathematics and Centre of Membrane Proteins and Receptors (COMPARE), University of Birmingham
5. Laboratory of Experimental Biophysics, Institute of Physics, EPFL
6. Randall Centre for Cell and Molecular Biophysics, King’s College London
- Keywords
Single molecule localisation microscopy (SMLM), Photoactivated localisation microscopy (PALM), Multiple blinking, Spatio-temporal point patterns
- Abstract text
Summary
Photoactivated localisation microscopy (PALM) produces an array of localisation coordinates by means of photoactivatable fluorescent proteins. However, observations are subject to fluorophore multiple-blinking and each protein is included in the dataset an unknown number of times at different positions, due to localisation error. This causes artificial clustering to be observed in the data. We present a workflow using calibration-free estimation of blinking dynamics and model-based clustering, to produce a corrected set of localisation coordinates now representing the true underlying fluorophore locations with enhanced localisation precision.
Introduction
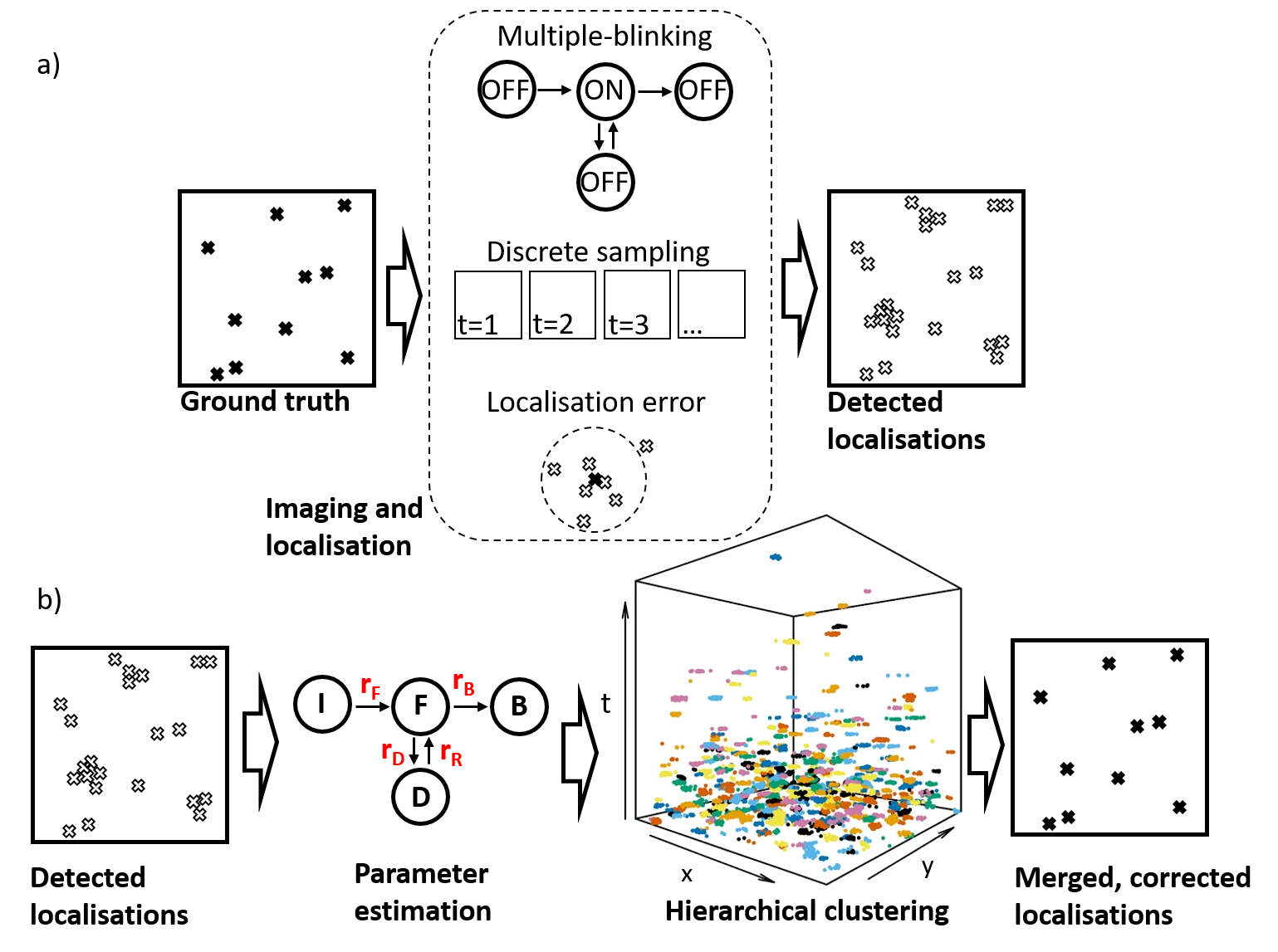
Single molecule localisation microscopy (SMLM) methods, such as PALM, circumvent the diffraction limit of light by separating fluorophore detections in time through stochastic activation and photobleaching, and then localizing the resulting sparse distribution of point spread functions [1]. The resulting point-pattern is a purported realisation of the underlying ground truth positions of the fluorophores, but is corrupted by a number of artefacts resulting from the photophysical behaviour of the probes as well as the imaging and localisation steps. Most problematic is the multiple appearance (multiple-blinking) problem where fluorophores undergo multiple on-off cycles before permanently bleaching, combined with the discretization effects that result from observing fluorescent signals on discrete camera frames [2]. The multiple-blinking problem results in data sets that are artificially clustered and overly populated (Figure 1a). As such, quantitative cluster analysis of SMLM data, in particular testing for spatial randomness of the underlying fluorophores, remains a challenge.
Methods/Material
In this work, we present a new method (Figure 1b) for correction of multiple-blinking artefacts in PALM data, which estimates, directly from the sample data set, the parameters of a realistic model of fluorescent protein photophysics [3]. Cluster analysis of the spatio-temporal (x,y,t,σ) data set then allows computation of the marginal likelihood of any given blink-merge proposal, under a full generative model for the data. We select the most likely of several proposals generated using a customised hierarchical clustering algorithm. Finally, each blink cluster is consolidated into a single position, now free from multiple-blinking and with improved localisation precision. The overall effect is to convert the set of raw x,y,t,σ localisation data into a new set, x,y,σ, with enhanced resolution.
Results and Discussion
PALM is increasingly used in the biological sciences and owing to the properties of commonly used total internal reflection fluorescence (TIRF) illumination, the distributions of membrane proteins have been especially well studied. Despite this, because of artificial clustering resulting from multiple-blinking, the question of whether membrane proteins are randomly distributed or not has become increasingly contentious [4]. Using our validated method combined with subsequent testing of the corrected protein locations, we show that the adaptor protein Linker for Activation of T cells (LAT) is clustered in the plasma membrane of CD4+ Helper T cell lines after the formation of an artificial immunological synapse [5,6] against an activating, antibody-coated surface. However, subsequent Bayesian cluster analysis [7,8] shows the clustering properties to be dependent on its macro-scale location within the synapse and on the presence of intracellular phosphorylatable tyrosine residues which mediate protein binding. We now propose that PALM, combined with the method we present here, can be used to test for spatial randomness in other membrane protein species.
Conclusion
In conclusion, our method allows for accurate recovery of ground-truth fluorophore positions, with enhanced precision, from PALM data sets subjected to multiple-blinking artefacts. For the first time, these corrected sets are of sufficient quality to allow accurate cluster analysis and the statistical testing for complete spatial randomness. We therefore believe that PALM combined with our method will be a valuable tool for addressing questions on the existence, determinants and functions of protein nanoscale clustering.
Figure 1: Illustration of our workflow. a) During PALM image acquisition and subsequent localisation steps, the ground-truth protein positions are corrupted by multiple-blinking in combination with discretisation by the camera frames and scrambling by the localisation uncertainty, resulting in a data set which is over-populated and over-clustered. b) Our algorithm takes as input x,y,t,σ data and estimates the rate parameters of a 4-state photophysical model, from which it derives the total number of molecules in the ROI. This is then used as input to a hierarchical clustering step (experimental data shown with colours representing the clusters found), after which clusters are merged to their centres, creating a new dataset free from multiple-blinking and with enhanced localisation precision.
- References
1. Betzig, E. et al. Imaging Intracellular Fluorescent Proteins at Nanometer Resolution. Science 313, 1642-1645 (2006).
2. Annibale, P., Scarselli, M., Kodiyan, A. & Radenovic, A. Photoactivatable Fluorescent Protein mEos2 Displays Repeated Photoactivation after a Long-Lived Dark State in the Red Photoconverted Form. The Journal of Physical Chemistry Letters 1, 1506-1510 (2010).
3. Jensen, L.G., Williamson, D.J. & Hahn, U. Semiparametric point process modelling of blinking artefacts in PALM. BiorXiv (2021).
4. Rossboth, B. et al. TCRs are randomly distributed on the plasma membrane of resting antigen-experienced T cells. Nature Immunology 19, 821-827 (2018).
5. Williamson, D.J. et al. Pre-existing clusters of the adaptor Lat do not participate in early T cell signaling events. Nat Immunol 12, 655-662 (2011).
6. Lillemeier, B.F. et al. TCR and Lat are expressed on separate protein islands on T cell membranes and concatenate during activation. Nature Immunology 11, 90-96 (2010).
7. Rubin-Delanchy, P. et al. Bayesian cluster identification in single-molecule localisation microscopy data. Nature Methods 12, 1072-1076 (2015).
8. Griffié, J. et al. A Bayesian cluster analysis method for single-molecule localisation microscopy data. Nature Protocols 11, 2499-2514 (2016).