Chemical Survey for Lithium-Ion Battery Electrode by ToF-SIMS Attached Xe Plasma FIB-SEM
- Abstract number
- 297
- Presentation Form
- Poster
- Corresponding Email
- [email protected]
- Session
- Poster Session 3
- Authors
- Xuhui Yao (1), Tomáš Šamořil (5), Jiří Dluhoš (5), John F. Watts (2), Zhijia Du (3), Bohang Song (4), S. Ravi P. Silva (1), Tan Sui (2), Yunlong Zhao (1, 6)
- Affiliations
-
1. Advanced Technology Institute, University of Surrey
2. Department of Mechanical Engineering Sciences, University of Surrey
3. Energy and Transportation Science Division, Oak Ridge National Laboratory
4. Neutron Scattering Division, Oak Ridge National Laboratory
5. TESCAN ORSAY HOLDING, a.s., Libušina tř
6. National Physical Laboratory
- Keywords
PFIB-SEM/ToF-SIMS system, Cross-sectional interface; Chemical survey; Lithium-ion battery electrode
- Abstract text
The focused ion beam with scanning electron microscopy (FIB-SEM) technique provides more versatility on sample preparation and cross-sectional interface observation. The time-of-flight secondary-ion mass spectrometry (ToF-SIMS) technique can achieve the chemical investigation with the features of high sensitivity, high efficiency and preservation of the sample spatial information.[1-2] Although the individual FIB-SEM and ToF-SIMS characterisation method has been widely applied, the combination of two techniques in one system has not attracted widespread attention in the battery community.[3-4] Integration of two techniques in one system enables the chemical survey for the cross-sectional interface of lithium-ion battery electrodes, not only featuring higher sensitivity and extended depth resolution but also avoiding the side reactions caused by the ambient atmosphere during the sample transfer process.[5-6]
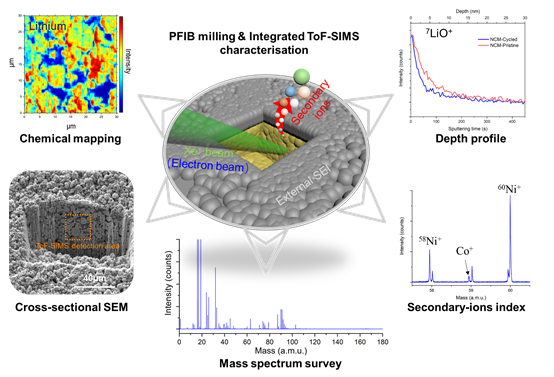
Here, we described the experimental operations and analysed both cathode and anode electrodes in pristine and after cycles. For the implementation, the integrated system was composed of the plasma FIB (PFIB) platform with Xe ion source (TESCAN SOLARIS X) and high-resolution orthogonal scanning ToF-SIMS analyser (H-TOF type from TOFWERK AG), which is developed collaboratively by EMPA (Swiss Federal Laboratories for Materials Science and Technology, Thun) and TESCAN company. (Brno, CZ).[7] The physical sputtering by ion beam was the source for both PFIB milling and ToF-SIMS characterisation. The maximal possible real resolution of ToF-SIMS characterisation is limited by PFIB spot size which depends on acceleration voltage and ion beam current. The incident angle of the primary Xe beam was 55° degree off the normal. Both positive and negative modes were applied on cathode and anode samples to achieve better sensitivities for positive and negative secondary ions, respectively. Through this setup, the SEM image and chemical information were observed directly on the cross-sectional interface of electrodes, possessing the ability to investigate the inhomogeneous distribution and degradation products inside the electrodes (Figure 1). As shown in the chemical mapping, the inhomogeneous distribution of lithium can be identified. The degradation products and the migration of transition metals can be observed on the mass spectrum after the indexing even at trace level. We believe this PFIB-SEM/ToF-SIMS system could further broaden the perception of the characterisation method, be of substantial interest and inspiration to the researchers in the battery community.
Figure 1. ToF-SIMS attached PFIB-SEM setup and its applications on battery characterisation. The schematic diagram of the sample is under integrated ToF-SIMS characterisation progress for the cross-sectional interface obtained by the PFIB milling.
- References
[1] B. Chait, and K. Standing, Int. J. Mass Spectrom. Ion Phys. 40 (2) (1981) 185,
[2] J. Pisonero et al., J. Anal. At. Spectrom. 24 (9) (2009) 1145,
[3] M.-S. Song et al., J. Mater. Chem. A 2 (3) (2014) 631,
[4] J. T. Lee et al., Carbon 52 (2013) 388,
[5] T. Sui et al., Nano Energy 17 (2015) 254,
[6] D. J. Miller et al., Microsc. Microanal. 24 (S1) (2018) 370,
[7] J. A. Whitby et al., Advances in Materials Science and Engineering 2012 (2012)