Caught in transition: Nanoscale analysis and molecular level characterisation of collagen mineralisation by complementary use of electron microscopy and in situ Raman microspectroscopy
- Abstract number
- 258
- Presentation Form
- Poster Flash Talk + Poster
- Corresponding Email
- [email protected]
- Session
- Stream 2: EMAG - In-situ microscopy
- Authors
- Emma Tong (4), Professor Roland Kroger (4), Dr Brian Wingender (3), Professor Laurie Gower (2), Dr Julia Parker (1)
- Affiliations
-
1. Diamond Light Source
2. University of Florida
3. University of North Florida
4. University of York
- Keywords
Biomineralisation Collagen PILP Electron Microscopy Raman Spectroscopy
- Abstract text
Bone is a fascinating and critically important biocomposite combining hardness and toughness through its hierarchical organisation facilitated by the nano-level organisation of an inorganic mineral [1], hydroxyapatite (HAp), and a protein, collagen type I, along with a minor fraction of non-collagenous proteins (NCP’s) and polysaccharides that affect the bone formation process largely due to their calcium binding potential. Despite intensive research the mechanism behind the mineralisation of the collagen matrix constituted of fibrils with approximately 100 nm diameter, is still a central problem in the field of bone research. Current understanding is that the mineral formation occurs via an amorphous precursor phase that infiltrates the collagen fibrils and subsequently crystallises [2, 3] However, the fundamental details of collagen infiltration and crystal growth remain controversial. A key problem is the understanding of the infiltration and mineralisation dynamics which we addressed by using ex situ transmission electron microscopy (TEM) in conjunction with electron diffraction for high-resolution analysis of the mineral complemented by novel in situ Raman microspectroscopy providing insights into the molecular level transport and transformation processes of the phosphate precursor phases. This approach allowed us to study the time dependence of precursor transport, intermediate phase formation and subsequent mineral growth using a model system of collagen mineralisation, namely the polymer induced liquid precursor (PILP) method. Our studies show that both intrafibrillar and extrafibrillar mineralisation occurs via an amorphous calcium phosphate (ACP) as most likely octacalcium phosphate (OCP) to HAp transformation in a time frame of approximately two hours whereas the complete mineralisation of collagen happened over a period of nine hours. Hence a rapid mineral phase transformation precedes the subsequent slower mineral growth process.
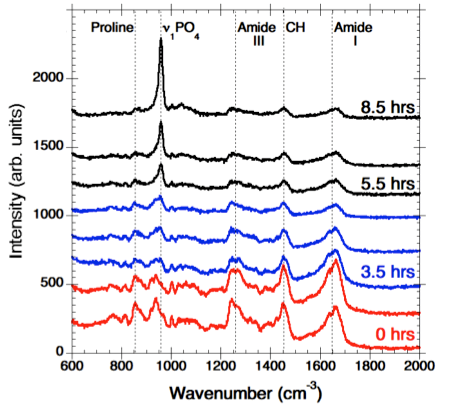
In order to identify the molecular level processes involved in the precursor transport and phase transformation, we have used an in vitro model system, the PILP process, by which collagen is mineralised in both intra- and extra-fibrillar spaces [2]. This leads to a nanostructural organisation that emulates key aspects of bone formation. Our in vitro model system employs a process-directing polymer as a biogenic analogue to the NCP’s found in native bone formation. Using a bespoke in situ heated liquid cell to maintain physiological temperatures within a Raman spectrometer, we have examined collagen mineralisation in real time, and observed peak development indicative of the transition from an amorphous precursor phase, ACP/OCP through to the formation of hydroxyapatite (HAp) crystals (Figure 1).
Figure 1. Time-resolved Raman spectra showing PO4 peak shift and evolution. The spectra highlighted in red shows the first stages of nucleation. The series of spectra highlighted in blue show the transformation from ACP/OCP to HAp. The black spectra show the increase of intensity in the PO4 peak indicating continuous transformation and growth of HAp crystals.
Furthermore, the kinetics and mechanisms of the transformation from ACP/OCP to HAp was studied using the Avrami model assuming that the fraction of mineralisation is given by
Φ = 1 − exp (−ktⁿ ) Eq.1
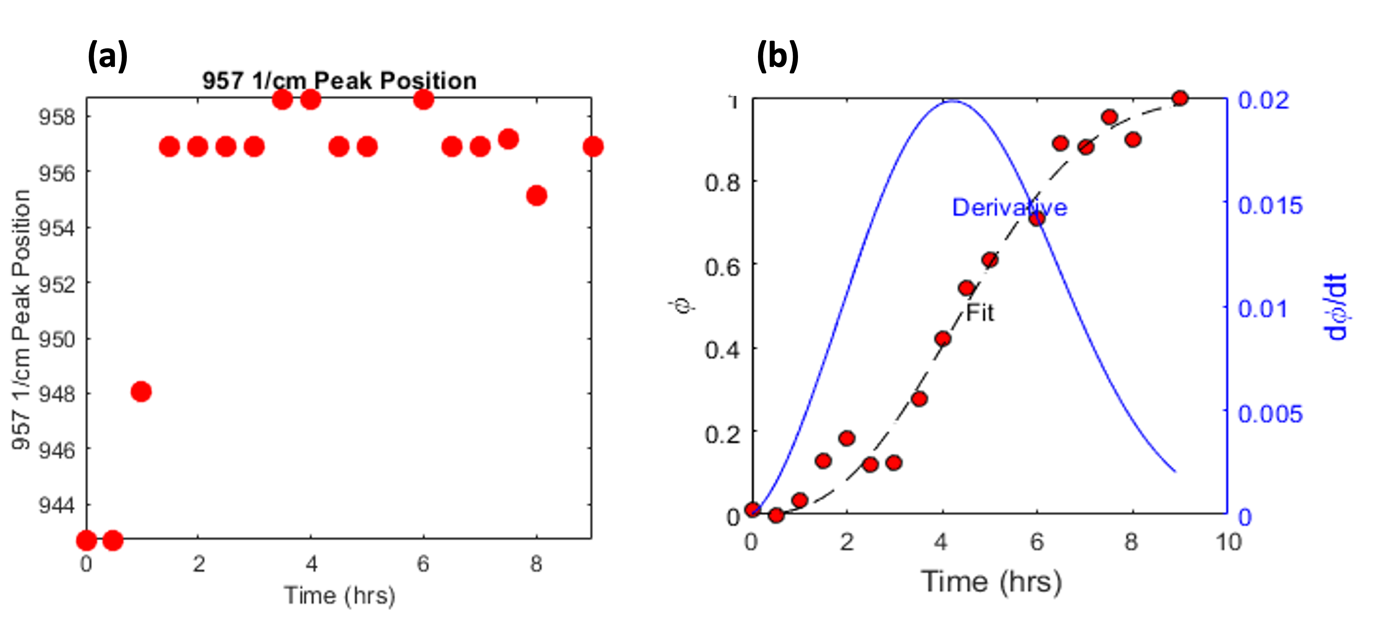
where the parameter k gives information on nucleation density and growth rates and n provides information on the dimensionality of the growth and the possible impact of diffusion. Observations show that the goodness of fit is satisfactory indicating that the transformation process follows Avrami type kinetics. We see a peak shift at approximately 1 hour indicating the transition between precursor phases (ACP/OCP) to HAp (Figure 2a). The derivative shows the maximum rate of transformation from ACP/OCP to HAp is at 4 hours (Figure 2b) whereas the transition phases can be observed at 1 hour indicating a rapid transformation to a mineral phase.
Figure 2. a) Time-resolved data showing the shift of the PO4 peak position. A sharp shift in the peak position from ACP942-948/OCP956 can be observed at approximately 1 hour. The peak of the position shifts again at approximately 1.5-2 hours from OCP956 to HAp958 where the peak position reaches a plateau once mineralisation has occurred. b) The derivative shows the maximum rate of transformation from ACP/OCP to HAp is at 4 hours.
Based on the values that Wong and Czernuszka give of n > 3 to either zero nucleation (n = 3), decreasing nucleation rate (n = 3–4), or constant nucleation rate (n = 4) for solvent mediated re-dissolution and re-crystallization processes [4]. Values below 3 indicate diffusion-controlled growth. Observations in the data (k = 0.014995 n = 2.5534) indicate a diffusion-controlled growth followed by a rapid and constant nucleation crystal growth.
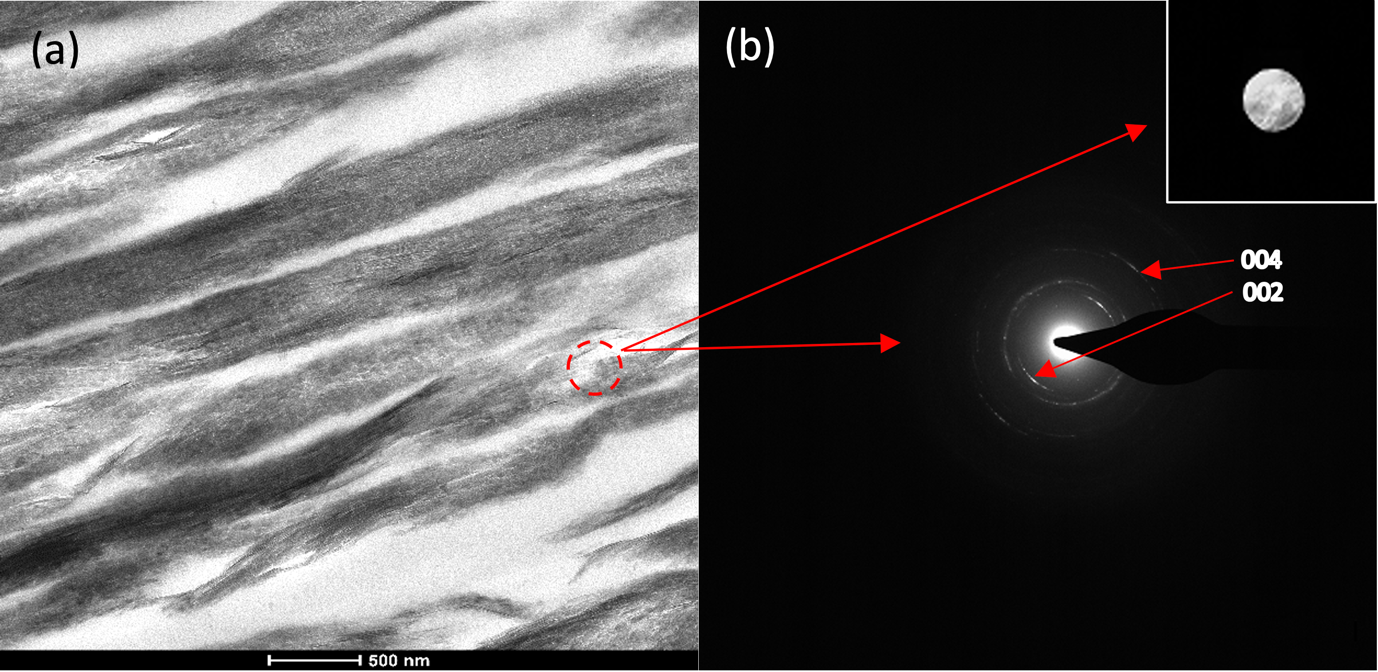
A detailed analysis of the resulting mineralised collagen was performed by TEM. Bright field TEM (BFTEM) images show densely packed, aligned collagen fibrils (Figure 3a). The characteristic d-banding of the collagen fibrils (indicative of the periodic arrangement of the gap- and overlap regions within the collagen fibres) can be clearly observed (Figure 3a). TEM images and selected area electron diffraction (SAED) patterns (Fig. 3a and 3b) are consistent with those for hydroxyapatite crystals. The (002) and the (004) reflection arcs can be observed in the diffraction patterns indicating that the [001] c-axis of the HAp crystals are roughly aligned with the collagen fibril axis within an angular range of approx. ±20˚ (Fig. 3b).
Figure 3. a) BFTEM image of mineralised collagen fibre. b) Selected area diffraction pattern of mineralised collagen showing the (002) and (004) reflection arc.
Furthermore, the BFTEM images revealed the coexistence of two crystal morphologies, platelet and needle like in shape (Fig. 4a and 4b).
Figure 4. a) BFTEM image of a mineralised collagen fibre showing the coexistence of two crystal morphologies. b) The characteristic collagen D- banding can be seen.
Our findings indicate that we can observe both intrafibrillar and extrafibrillar mineralisation akin to that observed in native bone. We have shown that using the Avrami model we can further study the kinetics of transformation during the mineralisation process. This work further allows for the quantitative characterisation of the kinetics of precursor infiltration and crystallisation, which are strongly dependent on the choice of polymer used for calcium and phosphate transport and opens up new avenues for a full understanding of bone mineralisation.
- References
- Reznikov, N., Bilton, M., Lari, L., Stevens, MM., Kroger, R. (2018). Fractal-like hierarchical organization of bone begins at the nanoscale. Science, 360.
- Gower, LB. (2008). Biomimetic Model Systems for Investigating the Amorphous Precursor Pathway and Its Role in Biomineralization. Chemical Reviews, 108 (11), 4551-4627.
- Olszta, M., Cheng, XG., Jee, SS., Kumar, R., Kim, YY., Kaufman, MJ., Douglas, EP., Gower, LB. (2007). Bone structure and Formation: A new perspective. Materials Science and Engineering, 58 (3-5), 77-116. https://doi.org/10.1016/j.mser.2007.05.001.
- Wong, ATC and Czernuszka, JT. (1993). Transformation behaviour of calcium phosphate 1. Theory and modelling. Colloids and Surfaces A: Physiochemical and Engineering Aspects, 78, 245-253.