Automating 3D Imaging of Heterogeneous Inorganic Nanoparticles
- Abstract number
- 124
- Presentation Form
- Submitted Talk
- Corresponding Email
- [email protected]
- Session
- Stream 2: EMAG - Automated Control, Advanced Data Processing
- Authors
- Dr Tom Slater (2), Dr Yi-Chi Wang (1, 6), Mr James McCormack (3), Dr Gerard Leteba (4), Dr Jhon Quiroz (5), Dr Pedro Camargo (5), Prof Richard Palmer (3), Prof Sarah Haigh (6), Dr Chris Allen (2, 7)
- Affiliations
-
1. Beijing Institute of Nanoengergy & Nanosystems
2. Diamond Light Source
3. Swansea University
4. University of Cape Town
5. University of Helsinki
6. University of Manchester
7. University of Oxford
- Keywords
Nanoparticles, Scanning Transmission Electron Microscopy, Automation, 3D Imaging
- Abstract text
Three dimensional imaging of inorganic nanoparticles has developed rapidly since first experiments in the early 2000s (Koster et al., 2000; Midgley & Weyland, 2003). The advent of aberration correctors and developments in electron tomography have led to three dimensional reconstructions in which individual atoms can be straightforwardly resolved (Yang et al., 2017; Van Aert et al., 2013). However, tomography experiments have the twin drawbacks of long acquisition times (and therefore high electron fluences) and sampling of relatively few nanoparticles.
In contrast, three dimensional imaging of viruses and proteins has recently been developed in to an automated tool that is applied with very low fluences and samples many thousands of particles (Tan et al., 2016; Xie et al., 2020). Automated routines for both acquisition and processing of data, and the development of user-friendly software, has widely disseminated the technique and this has contributed to a boom in its use.
We aim to emulate the progress made in cryo-EM by developing automated workflows for 3D imaging of inorganic nanoparticles. This encompasses nanoparticle image acquisition, segmentation of individual nanoparticles and 3D reconstruction routines.
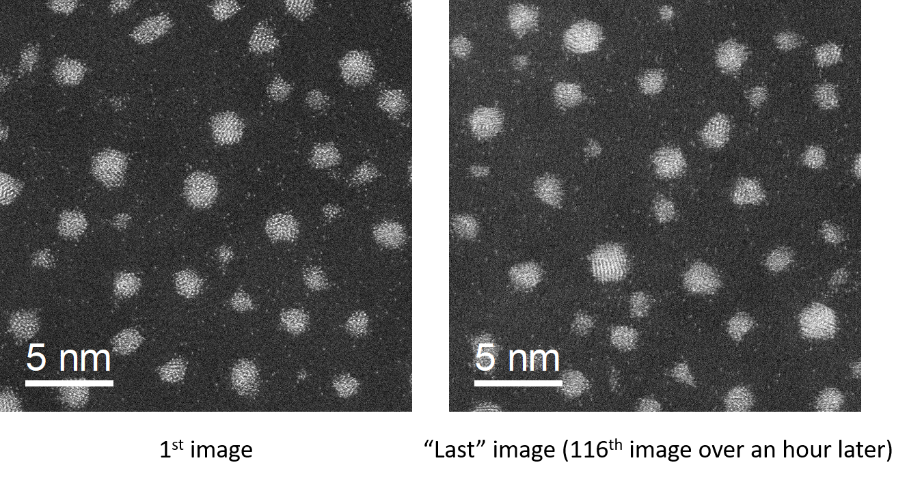
As a first step towards automated acquisition of high angle annular dark field (HAADF) images, we have developed a DigitalMicrograph script that allows automated acquisition of atomic resolution images (Slater, 2019). The script handles sample movement, automated checking of focus and re-focusing, checking the presence of nanoparticles and the acquisition of images. Hundreds of atomic resolution images can be acquired with no human input beyond initial setup (Figure 1).
Figure 1. HAADF-STEM images of sputtered Au clusters collected using the autoSTEM DigitalMicrograph script. The images were acquired over an hour apart with no operator input in-between.
Once images of nanoparticles are acquired, they are then segmented and analysed using the ParticleSpy python package (Slater, 2020), which we have developed to support our work on inorganic nanoparticles. ParticleSpy allows straightforward segmentation in an automated pipeline, using either simple thresholding methods or a machine-learning based trainable segmentation.
3D images are then obtained using the same single particle reconstruction algorithms used in structural biology. That is, each particle image is treated as a 2D projection of a 3D structure and, after determining the relative orientation of each projection, a simple backprojection algorithm is used to obtain the structure in 3D. The main issue in using this methodology for inorganic nanoparticles is that they are typically not as homogeneous in size and shape as viruses or proteins. To combat this issue, we cluster sets of particles images in terms of their properties (e.g. projected area, total HAADF intensity and a number of shape measures) using functions in the ParticleSpy package.
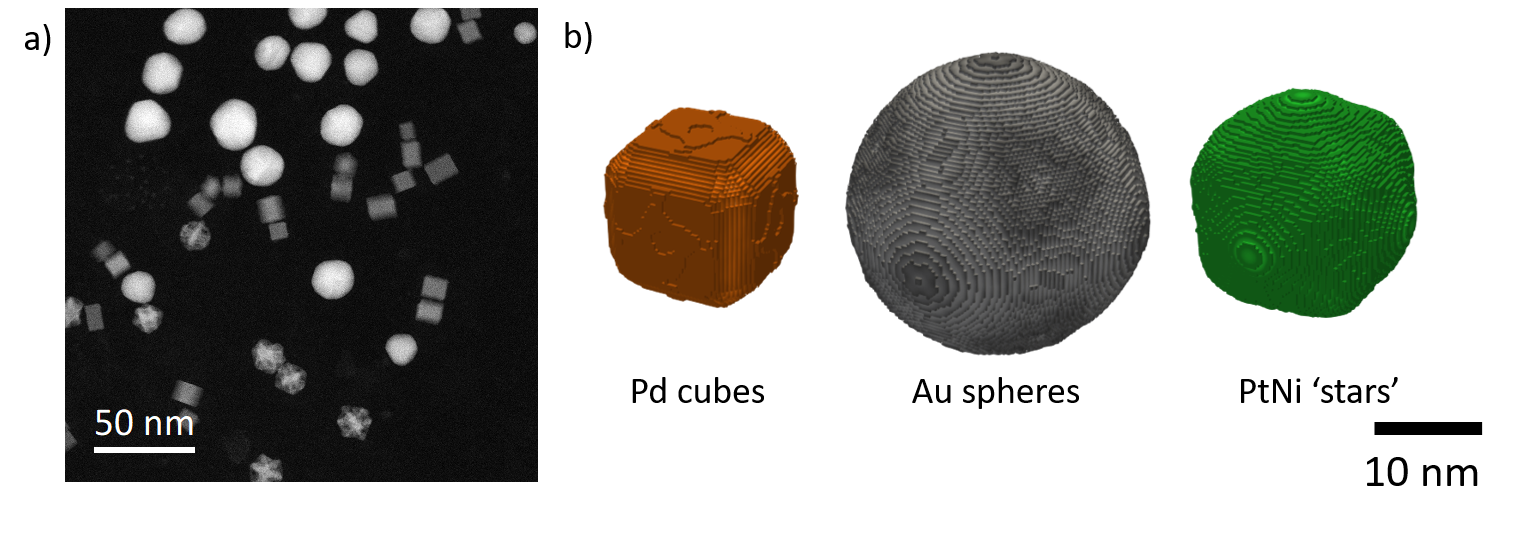
As an initial example, a sample containing three distinct particle populations was investigated to determine whether the workflow could distinguish between significantly different particles (Slater et al., 2020) (Figure 2a). A clustering algorithm was found that not only separated the three known populations, but also split these into sub-populations based on size and morphology differences. A 3D reconstruction of each sub-population could then be obtained using single particle reconstruction software (Figure 2).
Figure 2. a) ADF image of mixed nanoparticle population. b) Surface renderings pf the 3D reconstructions obtained via single particle reconstruction from a set of ADF mages equivalent to (a). The three distinct particle morphologies can be individually determined and reconstructed.
We have demonstrated a workflow that can reconstruct 3D images of nanoparticles that are not identical in morphology. We hope that this workflow will allow a wide spectrum of nanoparticles to be efficiently characterised in 3D with little human input. The presented methodology also meets the twin goal of lowering the fluence per particle and sampling a much larger number of nanoparticles when compared with STEM tomography. Our future goal is to push this technique to reconstruction with atomic-resolution, ushering in the possibility of acquiring the atomic structure of nanoparticle populations with one button click in the electron microscope.
- References
Koster, A. J., Ziese, U., Verkleij, A. J., Janssen, A. H. & De Jong, K. P. (2000). Three-dimensional transmission electron microscopy: a novel imaging and characterization technique with nanometer scale resolution for materials science. J. Phys. Chem. B 104, 9368–9370.
Midgley, P. A. & Weyland, M. (2003). 3D electron microscopy in the physical sciences: The development of Z-contrast and EFTEM tomography. In Ultramicroscopy vol. 96, 413–431.
Nord, M., Vullum, P. E., MacLaren, I., Tybell, T. & Holmestad, R. (2017). Atomap: a new software tool for the automated analysis of atomic resolution images using two-dimensional Gaussian fitting. Adv. Struct. Chem. Imaging 3, 9.
Slater, T. (2020). ePSIC-DLS/ParticleSpy: v0.4.1. https://zenodo.org/record/3763073.
Slater, T. J. A. (2019). autoSTEM. https://github.com/ePSIC-DLS/DM-Scripts/blob/master/autoSTEM Dialog.s.
Slater, T. J. A., Wang, Y. C., Leteba, G. M., Quiroz, J., Camargo, P. H. C., Haigh, S. J. & Allen, C. S. (2020). Automated Single-Particle Reconstruction of Heterogeneous Inorganic Nanoparticles. Microsc. Microanal. 26, 1168–1175.
Tan, Y. Z., Cheng, A., Potter, C. S. & Carragher, B. (2016). Automated data collection in single particle electron microscopy. Microscopy 65, 43–56.
Van Aert, S., De Backer, A., Martinez, G. T., Goris, B., Bals, S., Van Tendeloo, G. & Rosenauer, A. (2013). Procedure to count atoms with trustworthy single-atom sensitivity. Phys. Rev. B 87, 064107.
Xie, R., Chen, Y. X., Cai, J. M., Yang, Y. & Shen, H. Bin (2020). SPREAD: A Fully Automated Toolkit for Single-Particle Cryogenic Electron Microscopy Data 3D Reconstruction with Image-Network-Aided Orientation Assignment. J. Chem. Inf. Model. 60, 2614–2625.
Yang, Y., Chen, C.-C., Scott, M. C., Ophus, C., Xu, R., Pryor, A., Wu, L., Sun, F., Theis, W., Zhou, J., Eisenbach, M., Kent, P. R. C., Sabirianov, R. F., Zeng, H., Ercius, P. & Miao, J. (2017). Deciphering chemical order/disorder and material properties at the single-atom level. Nature 542, 75.