Advances in bio-TEM – Revolution or Evolution?
- Abstract number
- 163
- Presentation Form
- Poster
- Corresponding Email
- [email protected]
- Session
- Poster Session 4
- Authors
- Dr Christopher Edgcombe (1), A Sente (2)
- Affiliations
-
1. Dept of Physics, University of Cambridge
2. MRC Lab of Molecular Biology
- Keywords
Low-dose imaging; resolution revolution; beam-induced motion; beam/image shift; coma-free alignment; dynamic tilt correction.
- Abstract text
During the decade to 2014, biological imaging of single particles benefited from three major advances: the development of direct detectors, improved image processing and the increased stability of microscope systems. These improvements combined to produce the ‘resolution revolution’ described by Kühlbrand (2014), who mentions a resolution of 3.2 Å obtained then by cryo-microscopy. Some requirements for data collection to achieve resolution of better than 4 Å had been identified earlier by Glaeser et al (2011), who pointed out that it was advisable to align the microscope for minimum axial coma. Even with this alignment, disruptive off-axis coma could still occur due to beam tilt from several sources including the helical motion of electrons in the field of the objective lens.
Within the last two years, further advances, many of them pioneered at the Medical Research Council Lab for Molecular Biology (LMB), have improved the resolution of cryo-microscopy to substantially better than that achieved in 2014. Among the new developments are:
- The motion of images at the onset of irradiation (‘beam-induced motion’) has been traced to buckling of the ice film and has been suppressed by using a new design of substrate. As a result, specimen maps taken for successive doses can be extrapolated to zero dose (Naydenova et al 2020).
- For imaging sub-regions of the field, separated by distances of the order of micrometres, data can be collected substantially faster by shifting the beam than by moving the stage. However, this beam shift produces coma and astigmatism, as forewarned by Glaeser et al (2011). The coma can be corrected by adjusting the beam tilt dynamically at each position off the coma-free axis (Weis & Hagen 2020). The procedure is remarkably effective in improving the final resolution after processing, suggesting that the simple aberration theory on which it is based applies more accurately than might be expected.
- The first contributions to the wave aberration come from the objective lens defocus Δz and 3rd-order spherical aberration Cs. For values typical of cryo-microscopy, the aberration is dominated by the contribution from Δz. It thus becomes important to measure changes in Δz both dynamically and accurately and also to minimise its spread due to chromatic aberration and beam energy spread. Further attention to the illumination system can reduce fringing and maximise the information limit.
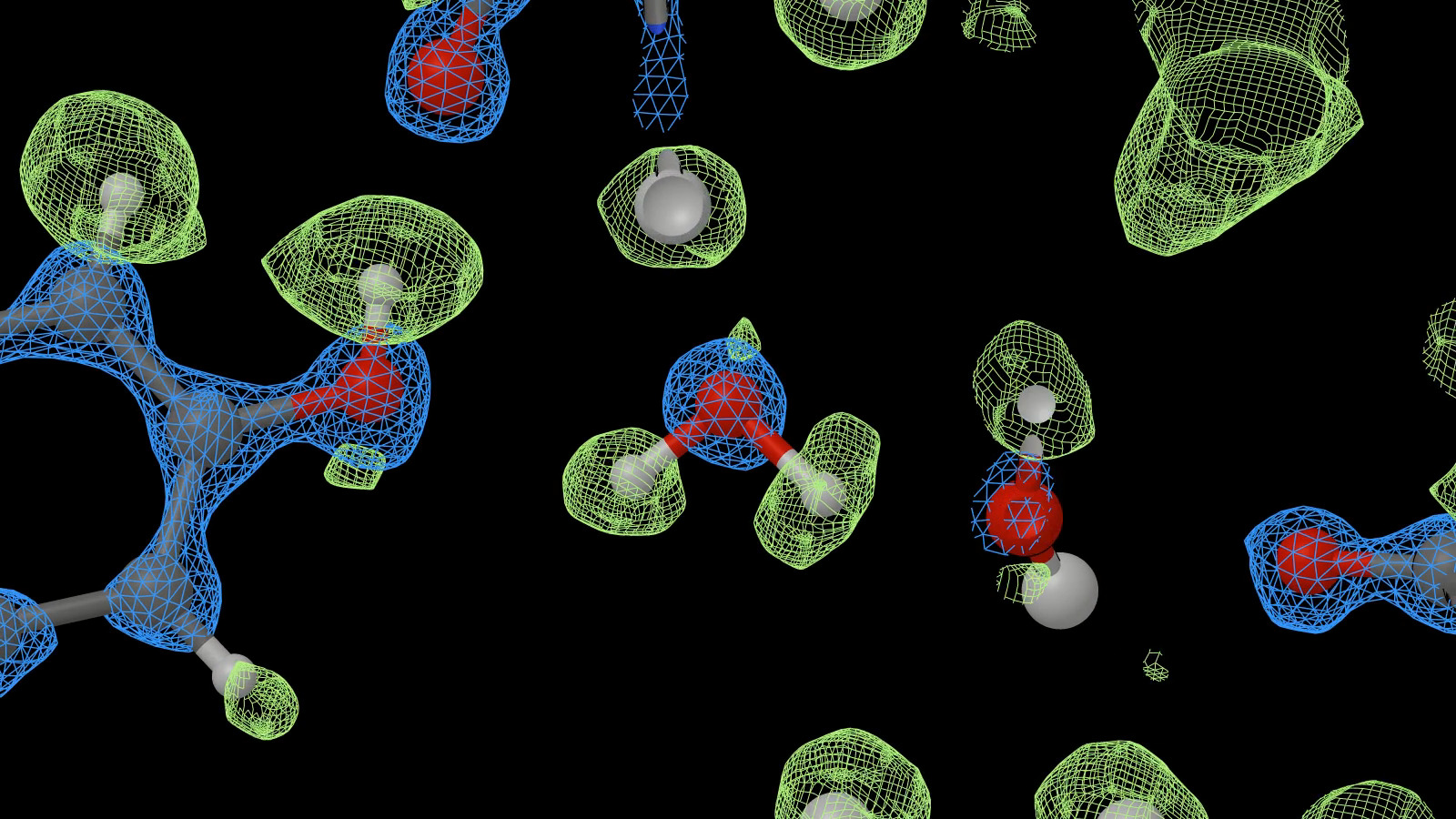
- A recent test by members of the MRC LMB, Thermo Fisher Scientific, University of Oxford, Vrije Universiteit Brussels, and Cambridge University Dept of Biochemistry (Nakane et al 2020) used a Krios microscope with a cold field-emission source, a new post-projector energy filter and a Falcon-4 camera. The system collected 3370 videos of mouse apoferritin within 36 hours and from a final set of 363126 particles achieved a resolution of 1.22 Å (Fig. 1).
This new landmark in resolution results from improvements in many aspects of the microscopy process. Further possibilities remain to be investigated, such as the feasibility of obtaining similar resolution at a beam voltage of 100 kV, which would substantially reduce the costs of the microscope system but may require further development of detectors.
The resolution revolution rolls on!
Fig. 1. Detail from a density map of mouse apoferritin, showing two trapped water molecules at the centre of the image (from a video produced by MRC LMB; see also Nakane et al, 2020).
- References
Glaeser, Typke, Tiemeijer, Pulokas and Cheng, J. Structural Biology 174 (2011) 1-10; doi: 10.1016/j.jsb.2010.12.005
Kühlbrand, Science 343 (28 March 2014) 1443; doi 10.1126/science.1251652
Naydenova, Jia and Russo, Science 370 (6513), 223–226 (2020) 9 October 2020; doi 10.1126/science.abb7927
Nakane, Kotecha, Sente, McMullan, Masiulis, Brown, Grigoras, Malinauskaite, Malinauskas, Miehling, Uchański, Yu, Karia, Pechnikova, de Jong, Keizer, Bischoff, McCormack, Tiemeijer, Hardwick, Chirgadze, Murshudov, Aricescu & Scheres, Nature 567 (5 November 2020) 152; doi 10.1038/s41586-020-2829-0
Weis and Hagen, Acta Cryst. (2020) D76, 724–728; doi 10.1107/S2059798320008347.