Accuracy of mass measurements by AFM
- Abstract number
- 215
- Presentation Form
- Poster
- Corresponding Email
- [email protected]
- Session
- Poster Session 1
- Authors
- Hans Gunstheimer (2, 3), Laura Gonzalez (2, 1), Gabriel König (2), Gotthold Fläschner (1), Daniel Müller (1), Patrick Frederix (2)
- Affiliations
-
1. ETH Zurich
2. Nanosurf AG
3. TU Ilmenau
- Keywords
AFM, cantilever, mass measurement, photothermal excitation, picobalance
- Abstract text
In this presentation we will discuss cell mass measurements by AFM, their accuracy as well as the influence of the mass position.
The mass of a cell is a fundamental measure of cells and dysregulation of the mass and mass development of cells can be related to many diseases (1). To relate the mass of the cell to its condition it is favorable to combine it with other techniques. Correlative mass measurements and optical microscopy is one obvious possibility, but also the combination with mechanical properties like adhesion or elasticity are of interest. Mass measurements by AFM fulfill these boundary conditions. It has been shown by this method that the cell mass fluctuates and increases over time for healthy cells (2). In contrast, virus-infected cells stop growing and energy depletion or blockage of water channels reduces cell mass fluctuations.
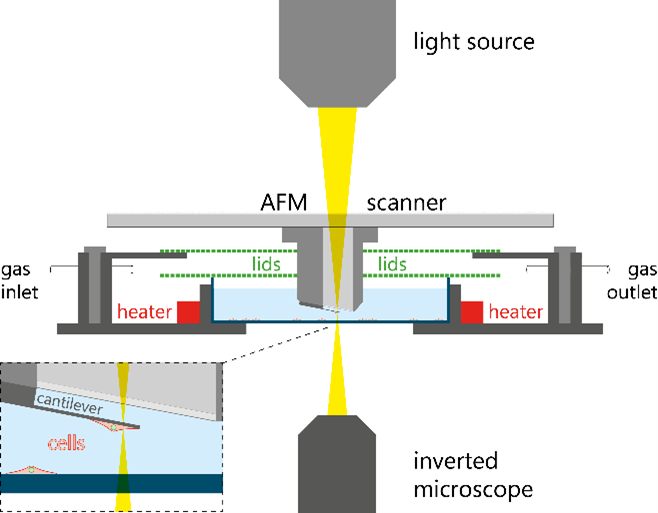
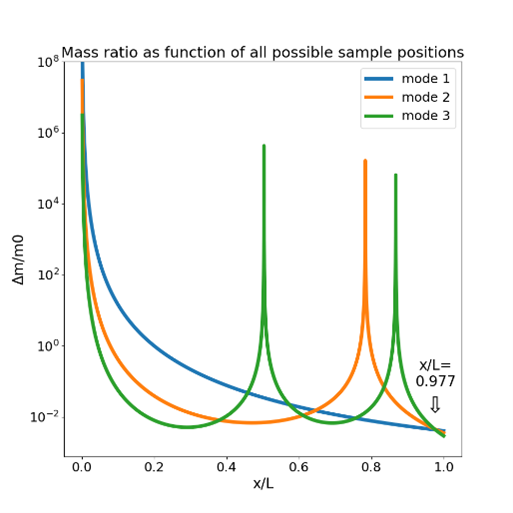
The mass measurement with an AFM is based on the drop in the cantilever’s resonance frequency when a mass is attached to it. Interestingly, this method reveals the cell’s total mass, including the contained water inside the cell rather than the buoyant mass, even with the cantilever immersed in water. In principle, the resonance frequency can be detected using the thermal spectrum of the cantilever, yet this limiting the accuracy and time resolution, particularly for stiff cantilevers. When the cantilever is directly actuated by photothermal excitation the frequency spectrum can be obtained with less noise and in combination with a phase-locked loop the resonance frequency can be tracked with millisecond resolution. The frequency drop not only depends on the mass, but also on the position of the mass on the cantilever. The position can be determined optically but this method is limited by the optical resolution of the microscope and even more so the difficult imaging conditions for low contrast cells on a cantilever. An alternative approach for the position determination uses multimode excitation of the cantilever at its normal modes using the Rayleigh-Ritz method (3).
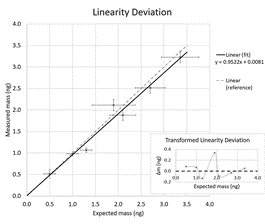
To determine the accuracy of this picobalance, particles of different sizes and densities were attached to a FluidFM® probe. The measured mass related well to the expected mass with an even narrower distribution than that derived the manufacturer specifications. The derived position by multimode excitation fitted the position of the suction nozzle of the FluidFM probe closely.
From the obtained results we conclude that the presented technology can be reliably used to measure the mass of cells and changes therein. Furthermore, the first results multimodal excitation of the cantilever seems a promising approach for automated determination of the position of the cell on the cantilever.
Schematic of AFM setup to measure the mass of cells attached to a cantilever
Colloidal particle attached to the opening of a tipless FluidFM micropipette with 8 µm opening
Measured mass versus expected mass. Every point represents the measurement of at least 25 particles.
Mass and position determination by multimodal excitation. Every curve shows the combinations of mass and position explaining the observed frequency shift for the first three modes. The intersection point (arrow) corresponds to the mass and position of the attached particle. The maxima of the curves are singularities located at oscillation nodes.
- References
- Lloyd, A. C. Cell 154, 1194 (2013)
- Martinez-Martin D. et al. D. Nature, 550, 500 (2017)
- Dohn S. et al. Rev. Sci. Instr. 78, 103303 (2007)